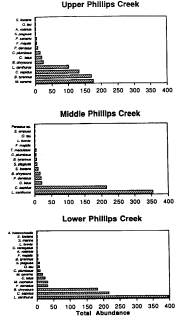
 Total nekton abundance, upper, middle, and lower Phillips Creek
stations, summer 1993.
Total nekton abundance, upper, middle, and lower Phillips Creek
stations, summer 1993.
Estuarine ecologists and resource managers recognize the importance of tidal marshes as nursery habitat for estuarine dependent finfish and crustaceans. Several authors have reviewed the considerable body of literature on this topic, which generally supports the concept that tidal marshes provide an abundance of food and serve as a predation refuge for marsh-dependent nekton. Boesch and Turner (1984) reviewed the nursery function of coastal salt marshes, citing the high primary productivity and spatially complex habitat as the primary reason for enhanced nursery function. These authors cite the relative lack of experimental studies to verify the predator avoidance function of salt marshes, but concur that limited observational data tend to support the refuge concept. Kneib (1986a) reviewed the role of the mummichog (Fundulus heteroclitus) in salt marsh trophic dynamics. This marsh-resident species is ubiquitous in Atlantic coast marshes ranging from Nova Scotia to northern Florida and functions as both predator and prey, depending on life-stage. The majority of life-history and production studies on F. heteroclitus have focused on the adult life stage; additional research efforts should focus on larval and juvenile mummichogs residing in the intertidal zone in order to fully understand the role of this species in salt marsh tropho-dynamics.
Thayer et al. (1979) reviewed the relative value of salt marshes as habitat for estuarine fishes and invertebrates in comparison to mangrove and seagrass ecosystems. These authors stressed the need for comparative and long-term studies of coastal marsh ecosystems and noted that the majority of research to date had been conducted on the Atlantic and Gulf coasts; little is known of fish utilization of west coast marsh/estuarine systems. Resource partitioning within specific habitats and the functional role of meiofuana were cited as areas deserving of investigation. Finally, the ability to relate commercial yields of estuarine dependent finfish and invertebrates to specific habitat types, was cited as a major area for future research.
Although the majority of habitat utilization studies have been conducted in salt marshes, it is recognized that oligohaline and tidal freshwater marshes also support economically important species. Odum et al. (1984) and Rozas and Hackney (1983) reviewed the community composition and nursery function of low-salinity wetland habitats along the U.S. Atlantic Coast. Anadromous and catadromous species including striped bass Morone saxatilis, American shad Alosa sapidisima, Atlantic sturgeon Acipenser oxyrhynchus and American eel Anguilla rostrata utilize tidal freshwater reaches of estuaries as spawning grounds and nurseries. Estuarine-dependent offshore spawners such as spot Leiostomus xanthurus, croaker Micropogonias undulatus and summer flounder Paralichthys dentatus will often migrate great distances as juveniles or adults into tidal freshwater reaches during summer to feed.
The majority of marsh habitat utilization research has focused on abundance/distribution studies or autecological studies on species of particular interest (i.e. Fundulus spp., Callinectes sapidus, etc.). Few studies have addressed marsh utilization on a community or ecosystem scale. The majority of studies have focused on the ecology of adult life stages, despite the recognition that tidal marshes are of particular importance as habitat for sub-adult (larval and juvenile ) stages. A significant body of work has been devoted to the importance of the marsh surface as a spawning area for marsh-resident species (i.e. Fundulus spp.). Relatively few studies have detailed feeding ecology and trophic dynamics of marsh-resident/dependent species and fewer still have investigated movements of marsh-resident/dependent species. Predator-prey interactions in tidal marshes have been investigated, however, environmental artifacts associated with experimentation in the marsh environment (i.e. wave action, sedimentation, cage effects) require caution in interpreting the results of field manipulations. In recent years, numerous studies have focused on specific selection of marsh microhabitats by resident and marsh-dependent species, and many have resulted in the development of new and innovative sampling techniques (Baltz et al., 1993; Hettler, 1989; Kneib, 1991; McIvor and Odum, 1988; Rozas, 1993). The drawback to this innovation, however, is that few of these studies can be effectively compared, due to a lack of methodological standardization. Thus, abundance and biomass estimates and faunal composition data resulting from most previous studies are of limited use in broadly characterizing marsh environments. Finally, a significant group of marsh habitat utilization studies focus on the use of reconstructed or altered coastal wetlands as habitat by marsh finfish and invertebrates (Gilmore et al., 1983; Harrington and Harrington, 1982; Neill and Turner, 1987; Rozas, 1992; Talbot et al., 1986). The degree of utilization by marsh-dependent nekton may ultimately determine whether a reconstructed or restored wetland habitat has achieved the functional equivalence of a natural marsh.
Coastal wetlands are the primary habitat for numerous estuarine-dependent and/or marsh resident species. Although secondary production and marsh trophic dynamics are generally considered to be community-level processes, several authors have investigated life history patterns, habitat utilization and production of individual finfish and invertebrate species, primarily those of ecological or economic significance. Merideth and Lotrich (1979) reported that productivity of F. heteroclitus from a Delaware salt marsh was among the highest reported for fishes (> 40.7 g m-2 year-1) and that sub-adults account for 78% of total annual production. Along the mid-Atlantic coast, F. heteroclitus co-occurs with other fundulids along an estuarine gradient from tidal freshwater to polyhaline marshes; however, the other species (F. diaphanus, F. luciae and F. majalis) rarely co-occur (Weisberg, 1986). Byrne (1978) and Kneib (1978) reported on the life history of the spotfin killifish (F. luciae), a small, secretive fundulid which occupies the higher intertidal zones of salt marshes. This species has been overlooked in previous abundance/distribution studies and is considered rare in many locales (Jorgensen, 1969; Richards and Bailey, 1967). This may be an artifact of sampling gear not designed to effectively sample infrequently flooded upper intertidal marshes (Shields and Hayes, 1983). More recent research suggests that this species is considerably more abundant than previously thought (Able et al., 1983; this volume Chapters II and IV). Cadigan and Fell (1985) reported on the reproduction, growth and food habits of the Atlantic silverside Menidia menidia from eastern Connecticut marshes. These authors documented the importance of coastal marsh systems as a nursery for silversides and postulate that this species may function as an important vector of energy transfer from tidal marshes to nearshore coastal waters. Several studies have focused on the population biology of spot Leiostomus xanthurus. This abundant marsh-dependent sciaenid is of significant economic value in the mid-Atlantic and southeast U.S. due to large commercial and recreational fisheries. Weinstein and Walters (1981) reported on the growth and production of L. xanthurus young-of-the-year (YOY) residing in tidal creek systems of the Cape Fear estuary, North Carolina. Spot spawn offshore in late fall and winter and post-larvae and juveniles begin to recruit into estuaries in late winter. Peak abundances of YOY are found in Spring. Mature spot migrate offshore in Fall, and may represent an important component of energy export to nearshore waters. Beckman and Dean (1984) reported that spot YOY recruit into North Inlet, South Carolina in three distinct cohorts at approximately monthly intervals and that immigration into the estuary occurs approximately 2 months following estimated date-of-hatch. Summer production of spot YOY residing in tidal marshes of the York River Estuary, Virginia was reported as six times greater than that reported for all size classes over the entire year (Weinstein, 1983; Weinstein et al., 1984). This strongly supports the concept of marsh-enhanced production of coastal fisheries.
Rickards (1968) reported on the ecology of juvenile tarpon Megalops atlanticus residing in Sapelo Island, Georgia salt marshes. Post-larval tarpon are primarily piscivorous and are in turn preyed upon by wading birds frequenting intertidal marsh pools and creeks. Ford and Mercer (1986) reported on the ecology of the american eel Anguilla rostrata from Great Sippewisset Marsh, Massachusetts. These authors suggest that a limited home range (< 100 m) and distribution of size classes is maintained by territoriality. Dagger-blade grass shrimp Palaemonetes pugio are a ubiquitous component of the salt marsh biota. Welsh (1975) investigated the role of P. pugio in trophic dynamics, nutrient cycling and decomposition at Bissel Cove, Rhode Island. Competition for resources is minimal in salt marsh ecosystems and grass shrimp are apparently able to maintain large populations and efficiently transfer detrital based energy to higher trophic levels. Alon and Stancyk (1982) stressed the environmental flexibility of P. pugio as a mechanism by which this species is able to occupy a variety of marsh habitats. Kneib (1987b) documented marsh surface use by larval and post-larval P. pugio, which had previously been assumed to not recruit to marsh surfaces until adults. Use of intertidal pit traps revealed that the upper intertidal zone may be the principal nursery area for grass shrimp. Consequently, little is known of the trophic role of sub-adult P. pugio in salt marsh systems.
The majority of research on salt marsh fisheries has been in the form of abundance/distribution studies. Kilby (1955) provided one of the earliest surveys of coastal marsh fish communities. He reported 75 species utilizing the brackish marshes in the vicinity of Cedar Key, Florida, the majority of which (85%) were of freshwater affinity. Dahlberg and Odum (1970) reported 70 species from marshes of Sapelo and St. Catherines Sounds, Georgia. Influx of juveniles into this area in late summer and fall resulted in a marked shift in species dominance. Richards and Castagna (1970) reported 70 species from back-barrier marshes and inlets along Virginia's Eastern Shore. Eleven species were considered residents and the remaining 59 were classified as migrants of varying degree. Norcross and Hata (1990) resurveyed the Virginia back-barrier marshes and lagoons, reporting 69 species. Additional abundance and species composition studies have been conducted in North Carolina (Weinstein, 1979), South Carolina (Cain and Dean, 1976) and New Jersey salt marshes (Roundtree and Able, 1992a). In Great Bay - Little Egg Harbor, New Jersey, Roundtree and Able (1993) documented significant diel differences in abundance of 15 marsh-dependent species. These differences were strongly influenced by season and life stage; for example, adult Atlantic silversides Menidia menidia were most abundant during the day in early summer, while young-of-year silversides were most abundant at night in late summer - fall. Studies of oligohaline marsh use by estuarine nekton suggest that these upper estuarine environments function as important nurseries for euryhaline species (Hackney and de la Cruz, 1981; Rozas and Hackney,1983; 1984). Long-term, multi-gear surveys of tidal freshwater nekton faunas are currently lacking. However, in Chapter V (this volume) the results of 10 years of research on nekton community ecology conducted at the Chickahominy River, Virginia are compiled, including a comprehensive list of species.
Several authors have attempted to study entire marsh fish communities, rather than select individual species, in order to more effectively understand the role of coastal marshes in enhancing fisheries production. Subrahmanyan and Drake (1975) classified the fish community of Northern Florida Juncus marshes into resident, foraging, and sporadic visitor components. The authors observed significant differences in species composition relative to tide stage. Subrahmanyan and Coultas (1980) investigated tide related movements and wide distribution of nektonic invertebrates and fishes in this same study area. Weinstein et al. (1980) documented a distinct ecotone at the mesohaline-polyhaline boundary in the Cape Fear River Estuary, North Carolina. This ecotone was responsible for a marked increase in species richness in high-salinity marshes. The marsh communities were relatively unaffected by changes in freshwater flow over the two years of the study period. Weinstein and Brooks (1983) compared nekton communities between a salt marsh creek and an adjacent seagrass meadow on the lower eastern shore of the Chesapeake Bay, Virginia. The salt marsh creek was characterized by a lower species richness than the seagrass meadow; however both habitats were frequented by opportunistic, wide ranging species with no obvious habitat preferences (ie. spot, blue crab, summer flounder). Peterson and Ross (1991) investigated faunal composition of tidal freshwater and oligohaline marsh/riverine habitats in Mississippi. They found greater species diversity and evenness in low-salinity environments relative to the mesohaline areas. They proposed that the low-salinity zones act as conduits between freshwater and estuarine habitats and are thus critical components of estuarine systems. Using intensive drop-sampling methodology and subsequent ordination techniques, Rakocinski et al. (1992) documented temporal and spatial changes in marsh community structure in response to large-scale and local environmental gradients in a Louisiana marsh system. Kushlan (1976) documented strong dependence upon seasonal fluctuations in water level in structuring finfish communities in the Florida Everglades, a non-tidal marsh system.
Coastal wetlands provide nursery habitat for resident and marsh-dependent finfish and invertebrate species, yet relatively few studies have focused specifically on use of estuarine marsh habitats by young-of-the-year. This is probably a consequence of the difficulty in sampling and accurately identifying larvae and juveniles in tidal marshes. Shenker and Dean (1979) used a modified channel net to collect larvae on ebb tides from a South Carolina intertidal marsh creek during winter. They reported 22 finfish species as present, in addition to grass shrimp and immature blue crabs. Spot Leoistomus xanthurus YOY numerically dominated the collections. Allen and Barker (1990) sampled larval fish in the same estuarine system using a benthic sled and determined that two distinct seasonal assemblages were present. The summer assemblage was dominated by gobies Gobiosoma sp. and anchovies Anchoa sp. whereas the winter assemblage was dominated by spot Leiostomus xanthurus and Atlantic croaker Micropogonias undulatus larvae. Rogers et al. (1984) investigated the influence of the spring freshet in determining the composition of YOY finfish in the Ogeechee River Estuary, Georgia. These investigators found that the dominant species utilizing the nursery were tolerant of high discharge and continued to use the estuary during the freshet period. Only one nursery species, the striped mullet Mugil cephalus was demonstrably affected by high flow conditions. Talbot and Able (1984) investigated the abundance and distribution of cyprinodontid larvae in high marsh microhabitats in New Jersey salt marshes. These authors suggested that the relative importance of high marshes as nursery areas for resident killifishes may have been previously underestimated.
Juvenile blue crabs Callinectes sapidus have received a considerable amount of attention in salt marsh systems primarily due to their importance as a commercial harvest. Orth and Montfrans (1987) found relatively few immature blue crabs in a lower Chesapeake bay tidal marsh creek relative to densities measured in an adjacent eelgrass Zostera marina bed. Thomas et al. (1990) found similar distribution of blue crabs from West Bay, Texas. Subsequent studies (Mense and Wenner, 1989; Wilson et al.,1990a) suggest that salt marsh creeks do not support high densities of juvenile blue crabs. In Louisiana coastal marshes, post-larval and juvenile brown shrimp Penaeus aztecus (which are spawned offshore) move into estuarine nursery areas in February and March. Peak densities of YOY in marshes occurs from March - May. Shrimp larvae may utilize a behaviorally mediated (via temperature and salinity) transport mechanism in order to take advantage of northward flowing currents associated with cold fronts to enhance recruitment success (Rogers et al., 1993).
The surface of tidal marshes may function as a critical spawning habitat for marsh resident finfishes (i.e. Fundulus spp.). A considerable body of literature details the use of the upper intertidal zone and the environmental mechanisms responsible for recruitment of resident fishes in this habitat. The mummichog Fundulus heteroclitus spawns in conjunction with spring tides and deposits its dessication-resistant eggs in the empty shells of the ribbed mussel Guekensia demissa or attaches them to the base of Spartina alterniflora stems and leaves (Able, 1984; Taylor and DiMichele, 1983). Reproductive condition (indicative of spawning readiness) in males and females is highest for several days coincident with full or new moons (Taylor and DiMichele, 1980). This tidal synchrony ensures deposition of eggs in the highest level of the upper intertidal, where they are least likely to be removed by tidal currents (Taylor et al.,1979). Egg hatching is triggered by appropriate conditions of submergence and low dissolved oxygen levels, but eggs may remain viable for up to 1 month exposed to air (Taylor et al., 1977; DiMichele and Taylor, 1980). Temperature and photoperiod have been implicated as environmental stimuli responsible for control of seasonal reproduction in F. heteroclitus (Taylor, 1986). Kneib (1986b) reported two distinct reproductive peaks in a Georgia population of F. heteroclitus. A similar bimodal pattern in annual reproductive activity occurs in F. heteroclitus populations from mainland marshes (but not back-barrier marshes) of Virginia's eastern shore (this volume, Chapter II). Kneib (1981) reported strong density-dependent effects on fecundity of F. heteroclitus from a North Carolina salt marsh. Using in situ manipulations, Kneib (1993) demonstrated that growth rate was positively associated and mortality rate negatively associated with tidal flooding for successive cohorts of F. heteroclitus larvae in a Sapelo Island, Georgia salt marsh. Tidal flooding may control the renewal rate of food resources (primarily harpacticoid copepods) in intertidal nursery microhabitats. Nursery use by a prior cohort was shown to have negative effects on survival of larvae in successive cohorts. Although not as intensively documented, marsh resident cyprinodontids from Gulf coast areas (F. pulvereus, F. grandis, Adinia xenica) apparently display similar reproductive periodicity and egg deposition strategies in response to a lunar cycle (Harrington, 1959; Greeley and MacGregor, 1983; Greeley 1984). Another group of marsh resident species, gobies (Gobiidae) utilize empty shells in oyster reefs in tidal marsh creeks as egg deposition sites. Competition for spawning sites among different co-existing gobiid species is apparently avoided by shell gape requirements specific to an individual species (Crabtree and Middaugh, 1982).
Feeding
p>
One of the primary nursery functions of tidal marshes is to provide an
abundance of food resources to swelling populations of YOY as they enter the
estuary and take up residence. Availability of food resources depends on
habitat type; the intertidal marsh surface will provide a different menu than
sub-tidal creek bottoms or the water column. Harrington and Harrington (1961)
documented feeding habits of marsh resident fishes (Cyprinodontidae) with
emphasis on their potential to control mosquito populations. Baker-Ditus
(1978) compared dietary overlap among three species of Fundulus (F.
majalis, F. heteroclitus and F. diaphanus) in the Patuxent River,
Maryland. She found that dietary overlap increased with increasing food
abundance. Weisberg et al. (1981) determined that mummichogs F.
heteroclitus were primarily daytime feeders and that feeding activity is
greatest at high tide, regardless of whether or not marsh surface areas were
inundated. Mummichogs preferentially moved up onto and fed upon the marsh
surface when this habitat was available. Food limitation was shown
experimentally to regulate maximum size of mummichog populations by affecting
individual growth rates, mortality and fecundity (Weisberg and Lotrich, 1986).
Kneib and Parker (1991) experimentally determined that natural prey
concentrations may be sub-optimal for mummichog larvae but not for spotfin
killifish residing on the surface of Sapelo Island, Georgia salt marshes.
Rozas and LaSalle (1990) documented habitat-specific feeding preferences for
the Gulf killifish Fundulus grandis in a Mississippi brackish marsh.
Killifish feeding in the intertidal zone consumed primarily fiddler crabs
Uca longisnalis and polychaetes. Fishes feeding in the subtidal
consumed mostly amphipods Corophium louisianum. In addition, killifish
with access to the marsh surface contained a greater volume of food, on
average.
Worgen and Fitzgerald (1981) compared diets of three sympatric species of sticklebacks (Gasterosteroidae) from the St. Lawrence River and concluded that diets were similar among the three species and that resource partitioning by food type and time were of little significance in explaining the co-existence of the three species.
The economic significance of marsh-dependent sciaenids has resulted in a number of studies of the dietary habits of these fishes as juveniles. Spot Leiostomus xanthurus change from planktivorous post-larvae to benthic feeders as juveniles and may consume 4.5% of their body weight per day. Gut fullness is greatest during high tide and immediately thereafter (Hodson et al., 1981; Archambault and Feller, 1991). The red drum Sciaenops ocellatus is perhaps the most important gamefish along the U.S. Gulf coast. Adult red drum enter Gulf coast marshes to feed throughout the year. During winter and spring, red drum are mainly piscivorous. During summer and fall, decapod crustaceans make up the majority of the diet of this species (Boothby and Avault, 1971). Juvenile red drum are abundant temporary residents in Gulf coast marshes, feeding primarily on benthic crustaceans and the young of other marsh dependent fishes (Bass and Avault, 1975). Another economically important sciaenid species, the spotted seatrout Cynoscion nebulosus, utilizes Gulf coast marshes to feed, primarily selecting small fishes and decapod crustaceans (Lorio and Schafer, 1965.). Silver perch Bairdiella chrysoura feed nocturnally in the lower intertidal zone during high tide (Kleypas and Dean, 1983). Rountree and Able (1992b) investigated the foraging habits of young-of-the-year Summer flounder Paralichthys dentatus from southern New Jersey salt marshes. Flounders preyed heavily on resident fishes (Menidia menidia, Fundulus spp.) and decapods (Palaemonetes vulgaris, Crangon septemspinosa). Analysis of gut fullness revealed that flounders undergo tide-related movements to take advantage of high prey concentrations within marsh creeks. Tarpon Megalops atlanticus provide an economically important sportfishery in the Southeastern U.S. The young of this species are often abundant in high marsh pools and rivulets where they prey upon copepods, ostracods, insects and small resident fishes. (Harrington and Harrington, 1960; Rickards, 1968). Heard (1975) investigated the feeding habits of white catfish Ictalurus catus from the North Newport River Estuary, Georgia and reported that this species was an opportunistic, omnivorous feeder, with small crustacea, primarily amphipods, dominating in the diet. Relatively few studies have examined the feeding habits of marsh dependent crustaceans. Ryer (1987) examined the feeding periodicity of blue crabs Callinectes sapidus in salt marsh creeks and an adjacent seagrass meadow in lower Chesapeake Bay, Virginia. In the marsh, feeding was related to the tidal cycle. Crabs had fullest guts at high tide; guts were least full immediately preceding the next high tide.
Perhaps the most important topic in recent marsh habitat utilization studies has been the determination of patterns of habitat selection by marsh resident and marsh dependent nekton. Restoration of degraded marshes and creation of artificial marshes has gained popularity as a mitigation tool in recent years, yet we still know relatively little about the way in which marsh dependent finfish and invertebrates select and utilize specific marsh habitats. Without detailed understanding of these habitat selection processes, the ability to develop artificial marshes which effectively support and enhance estuarine fisheries is lacking.
Marsh-resident species and marsh-dependent offshore spawners utilize the flooded salt marsh surface edge as a forage area during high tide (Hettler, 1989; Baltz et al., 1993). The marsh surface/creekbank ecotone is of similar importance as a refuge and forage area for nekton residing in tidal freshwater marshes along the mid-Atlantic coast (McIvor and Odum, 1988). The upper intertidal zone is of primary importance as a forage site and predation refuge for smaller, marsh resident species (primarily Cyprinodontidae and daggerblade grass shrimp Palaemonetes pugio (Kneib, 1984; 1987a). The sub-tidal areas of marsh creeks may support high densities of marsh resident and demersal marsh-dependent fish species and grass shrimp. In a comparison of salt marsh creeks, eelgrass Zostera marina beds, and sea lettuce Ulva lactuca as habitat for fishes and decapods in New Jersey estuaries, Sogard and Able (1991) reported highest densities from marsh creeks. In tidal freshwater marshes, nekton utilize dense beds of submerged vegetation in order to feed and to avoid predation (Rozas and Odum, 1987a; 1988). Nekton density was greatest in headwater (order 2) marsh creeks which contained denser SAV beds as compared to riverine (order 4) stations (Rozas and Odum, 1987c). In experiments where SAV was either added or removed from areas adjacent to creeks, grass shrimp Palaemonetes pugio densities increased, and decreased correspondingly. Fish densities did not change overall in response to the manipulations (Rozas and Odum, 1987b) Depositional creekbanks provide fishes with access to the marsh surface, where they may forage effectively and avoid predators. In addition, the depositional subtidal environment itself functions as a shallow refuge and provides an abundance of benthic invertebrate prey (McIvor and Odum, 1988). Nekton may also use small intertidal rivulets as corridors for accessing the marsh surface, although the areal extent of such rivulets in tidal marshes is minimal (Rozas et al., 1988).
Several recent studies have documented the importance of salt marsh habitat as a nursery area for juvenile penaeid shrimps. Brown shrimp Penaeus aztecus densities were significantly higher in Spartina marsh habitat as compared to adjacent non-vegetated areas in a Galveston, Texas salt marsh. White shrimp P. setiferus showed no preference for habitat type (Giles and Zamora, 1973; Zimmerman and Minello, 1984), and laboratory studies demonstrated that foraging Atlantic croaker select this species over P. aztecus (Minello and Zimmerman, 1985). Rozas and Reed (1993) found that penaid shrimp (P. aztecus and P. setiferus) numerically dominated nekton collections from "hummocky" Spartina marshes undergoing submergence in coastal Louisiana. Although in an advanced state of deterioration, the submerged marshes in this study retained their habitat function.
Juvenile and adult blue crabs Callinectes sapidus have been shown to use the intertidal zone of a Sapelo Island, Georgia salt marsh as a refuge and feeding area during Spring and Summer (Fitz and Weigert, 1991). Crabs were virtually absent from the marsh surface in Winter. Few studies have focused on the use of intertidal habitats by C. sapidus. Juvenile and adult blue crabs are also present on the surface of tidal freshwater marshes from Spring - Fall and adults overwinter in shallow subtidal areas of tidal freshwater marshes (pers. obs.).
Movements of marsh resident/dependent nekton has not been studied in great detail. Conventional mark and recapture techniques are often not applicable to small or sub-adult fishes or macrocrustaceans. Alternative marking techniques such as "sandblasting" with fluorescent pigments has been attempted on juvenile sciaenids (Arnoldi et al., 1974) but recapture rates within the estuary were extremely low. Buttner and Brattstom (1960) used fin-clipping techniques to determine return rates of Fundulus heteroclitus and Menidia menidia in a Long Island, New York salt marsh. They concluded that marsh resident fishes return to tributary creeks only as a function of localization of activity, and that no evidence of homing was detectable for these resident fishes. Adult mummichogs displayed a summer home range of < 36 m in a Delaware salt marsh. Fishes moved into low-salinity headwaters of the creek to overwinter. Winter activity of F. heteroclitus in upstream habitats was controlled primarily by temperature and photoperiod (Lotrich, 1975; Fritz et al., 1975). Rountree and Able (1993) tagged juvenile Summer flounder captured in southern New Jersey salt marsh creeks. They reported a recapture rate of 39% and estimated that the average period of creek use by flounders was 17 days with 100% emigration occurring within 50 days of release. Much additional work is needed to characterize localized movements and mass migrations of marsh-dependent fishes, of all life stages. Newer technology in the form of miniature tags which can be surgically implanted into very small individuals and ultrasonic tracking (Wirjoatmodjo and Pitcher, 1984) will undoubtably prove to be of great value in future studies of this type.
Sub-adult marsh dependent nekton function as both predators and prey in coastal wetlands. Marsh resident species, including killifishes and caridean shrimp, are clearly of importance as prey to a host of marsh-dependent predators, whether they be juveniles of offshore spawned species (Callinectes sapidus, Sciaenidae) or adventitious, occasional marine visitors to estuarine nursery areas.
Mummichog (Fundulus heteroclitus) predation may control the abundance and size distribution of marsh surface dwelling invertebrates including the gastropod Melampus bidentatus and the amphipod Orchestia grillus (Vince et al., 1976). Kneib (1987a) used in situ enclosure/exclosure experiments to demonstrate that sub-adult grass shrimp Palaemonetes pugio and mummichogs remain in shallow intertidal habitats at low tide in order to avoid predation from predatory adult mummichogs. Predation by wading birds (Ardeidae) and blue crabs has been shown experimentally to determine the size structure of F. heteroclitus populations (Kneib, 1982). Size-selective predation on mummichogs by wading birds and/or blue crabs may strongly determine the abundance, community structure and size distribution of marsh infauna. Blue crabs preferentially selected marsh periwinkles Littorina irrorata and gulf killifish Fundulus similis over infaunal prey (the ribbed mussel Geukensia demissa) in a Dauphin Island, Alabama salt marsh (West and Williams, 1986). Apparently, predation upon ribbed mussels requires greater energy expenditure due to excavation relative to the amount of energy expended by crabs to capture killifish or periwinkles. Blue crabs also function as prey in salt marsh habitats, particularly as juveniles. In a New Jersey tidal marsh creek, tethered juvenile blue crabs experienced predation rates in excess of 40% relative to crabs tethered in eelgrass Zostera marina or sea lettuce Ulva lactuca habitats (Wilson et al., 1990b). In mainland fringing marshes of the Virginia Coast Reserve, adult blue crabs are the dominant prey item consumed by YOY sandbar sharks Carcharinus plumbeus using salt marsh creeks as a nursery habitat (see Appendix I).
The role of submerged vegetation in providing a predation refuge for marsh dependent nekton has been examined with emphasis on survival of juvenile penaeid shrimps (Minello and Zimmerman, 1983; Minello et al., 1989.). Experiments with artificial vegetation demonstrated that inefficient marsh predators such as pinfish Lagodon romboides, southern flounder Paralichthys lethostigma and Atlantic Croaker Micropogonias undulatus experienced significant reduction in prey capture ability, whereas prey capture ability of superior predators such as red drum Sciaenops ocellatus and speckled trout Cynoscion nebulosus were unaffected by vegetative structure in the intertidal. Low water levels brought about by climatic and/or man induced alterations reduce access by penaeids to the intertidal zone and may result in increased mortality due to predation.
The inherent difficulty of sampling nekton from coastal marsh environments is a function of soft substrates and dense emergent vegetation. Various alternatives to the conventional "seine/trawl" methodologies have been developed and many are truly effective at obtaining quantitative or semi-quantitative samples. Unfortunately, the development of new technologies has led to a lack of standardization among habitat use studies. Nekton densities cannot be realistically compared among studies using alternative sampling techniques. In addition, nearly all gear types are selective for size or life-history characteristics. Consequently, few truly effective comparisons among marsh habitats have been made either on a local or regional scale.
Kjelson and Johnson (1973) developed a portable drop-net for the active collection of motile estuarine nekton. Drop nets or throw traps have been used successfully in obtaining quantitative estimates in several subsequent marsh habitat studies (Rakosinski et al., 1992; Baltz et al., 1993; Rozas and Odum, 1987a), and are among the most effective methodologies, although the surface area sampled is relatively small and disturbance of the site by the operator can affect sampling precision. Rogers (1985) described a small "push-otter trawl" designed specifically for use in shallow marsh areas. The trawl was intended for use on an airboat, but with minor modifications could be used with conventional shallow-draft skiffs given sufficient water depth. Herke et al. (1977) described a stationary trap designed for use on tidal weirs in semi-impounded marshes. Studies of nekton use of the flooded marsh surface have been conducted using intertidal pit traps (Kneib and Stiven, 1978; Kneib, 1984; Shields and Hayes, 1983; Talbot and Able, 1984). Typically, wire mesh baskets or shallow plastic containers are used to collect small fishes and nektonic invertebrates which reside on the marsh surface at low tide. These containers simulate the natural potholes and depressions present on the marsh surface which are utilized by sub-adult fishes and macrocrustaceans (Kneib, 1984). Rozas (1993) developed a bottomless lift net to sample the flooded marsh surface. Fishes trapped within the lift net are concentrated in a shallow pit located within the trap area as the tide recedes. Using this method, it is possible to obtain quantitative areal estimates of marsh-surface nekton. One of the most innovative and versatile sampling techniques to be developed is the flume net (McIvor and Odum, 1986) This passive semi-quantitative sampling device samples fish leaving the flooded surface of tidal marshes during receding tides. Fishes leaving the marsh surface pass through the mesh flume and are captured in a removable cod end deployed at the onset of slack tide. This gear type is effective at capturing those fishes which frequent the lower flooded intertidal to forage and do not remain on the surface of the marsh at low tide. Hettler (1989) used a block net to entrap fishes and macrocrustaceans leaving the flooded marsh surface at the creekbank ecotone in North Carolina. Kneib (1991) developed a quantitative device to sample resident and transient marsh surface nekton from the interior of flooded marshes, the flume weir. This is probably the most effective and truly quantitative gear type developed for use in tidal marshes thus far, however, it is costly and requires a large investment of man-hours.
The efficiency of conventional gear types (seine and trawl methodology ) has been tested for use in tidal marsh environments. Weinstein and Davis (1980) compared efficiencies (defined as percentage of the number of individuals captured from a known area of tidal creek) for seine and rotenone (5% Fish-Tox) samples from the Cape Fear River Estuary, North Carolina. They found that the range of efficiencies was greater for seine samples (61 - 78% vs. 30 - 58%) but that overall species richness was higher in rotenone samples. In a more recent study, Allen et al., (1992) found that a single seine haul was sufficient to estimate species richness, species rank and size distributions for a population of fishes in a South Carolina intertidal pool. Abundance was not reliably estimated in a single collection, however, and collection efficiency ranged from 7 - 91%. Due to seasonal differences in fish avoidance behavior, these investigators recommended that haul seine efficiencies be measured at least once a season in long-term studies of this type. An experimental fish enclosure is described by Dieter et al., (1991) for use in shallow pothole wetlands. This type of gear could easily be adapted for use in a tidal marsh environment and would be of use in studies of fish growth, enclosure/exclosure studies, or aquaculture research.
Alteration of coastal wetlands by impoundment, pipeline canal dredging and construction of drainage ditches has occurred extensively on the East, Gulf and West coasts of the United States. These activities alter the flooding characteristics of the marsh, which in turn may seriously affect sediment distribution patterns, geomorphology, plant community composition and the distribution and abundance of marsh-dependent finfish and invertebrates. In recent years, restoration of previously altered marshes and the construction of artificial marshes has gained popularity as a mitigation tool. The majority of these restoration/creation projects are undertaken without any specific design criteria regarding potential use of the new habitat by marsh-dependent finfish and invertebrates. Detailed understanding of the role of marsh microtopography, creekbank geomorphology and plant community composition with regard to use of the created habitat by marsh- dependent nekton would enhance the success of marsh restoration projects and ensure that they provide their intended nursery function.
Impoundment of an east-central Florida salt marsh was shown to drastically reduce the habitat quality and nursery function of the wetland (Harrington and Harrington, 1982). Before and after-impoundment surveys revealed that 16 species of fishes were absent following impoundment including Tarpon Megalops atlanticus and snook Centropomas undecimalis. Numbers of rainwater killifish Lucania parva, marsh killifish Fundulus confluentus and sheepshead minnows Cyprinodon variagatus were significantly reduced. Two species increased in abundance; the mosquitofish Gambusia affinis and the sailfin molly Poecilia latipinna. The resident marsh insect fauna was also impoverished following impoundment and cyclopoid copepods, once abundant, became rare. Dinoflagellate blooms became common in the impounded marsh and the diets of resident fishes switched from reliance on insects and zooplankton to reliance on vascular plant detritus and algae. Gilmore et al. (1983) compared the resident ichthyofauna of open and closed salt marsh impoundments in east-central Florida. They reported a depauparate ichthyofauna (12 species) and stressful environmental conditions for the closed marshes while the open impoundments were characterized by greater fish species richness (41 species) and extensive regrowth of marsh vegetation. Comparison of marshes ditched and impounded for mosquito control with unaltered marshes in New Jersey showed distinctly different fish assemblages (Talbot et al., 1986). Impounded marshes were characterized by freshwater/oligohaline species while unaltered marshes and marshes altered using open marsh water management (OMWM) techniques were characterized by a typical estuarine species assemblage. Distinct seasonal changes in the fish assemblages were observed in natural and OMWM sites, but not in ditched/impounded sites. Herke et al. (1987) reported 4 times greater abundance of brown shrimp Penaeus aztecus from natural marshes when compared to impounded brackish marshes in Marsh Island, Louisiana. Shrimp stayed longer in the impounded marsh and emigrated at a larger size. Neill and Turner (1987) compared fish habitat use in open and plugged backfilled canals in Louisiana coastal marshes. The mean number of migrant species was greater in open or semi-open canals; resident species dominated in plugged canals. Plugging canals reduced habitat value by rendering nursery areas located beyond plugs inaccessible. Rogers et al. (1992) evaluated the effectiveness of a marsh management plan in south-central Louisiana on fish community composition and salinity. Managed areas which were subject to periodic drawdown were characterized primarily by a resident (non-migrant) nekton fauna. Unmanaged areas supported a transient, marsh-dependent fauna. Rozas (1992) compared fish abundance and composition between natural marsh channels and man-made canals. He found no significant difference in overall abundance between habitat types. Tethering experiments revealed that predator encounter rates in the the two habitat types were similar, suggesting that the habitat value of canal bottoms approximates that of natural creek bottoms as slumping decreases the steepness of bottom profiles. Using replicate natural and transplanted dredge spoil marshes, Minello and Zimmerman (1992) tested the null hypothesis that transplanted marshes were functionally equivalent to natural marshes as nekton habitat in Texas. Densities of decapod crustacea (primarily dagger-blade grass shrimp Palaemonetes pugio and brown shrimp Penaeus aztecus) were significantly higher in natural marshes. Fish densities, however, were comparable between the two habitat types. Decapods may have been responding to low densities of prey organisms (small benthic invertebrates) on the surface of transplanted marshes, whereas fishes may rely on marshes primarily for protective cover, which is is provided at both natural and transplanted sites. A better understanding of the role of intermediate level (invertebrate) trophic pathways is needed to validate this somewhat contradictory conclusion.
Numerous questions remain to be answered regarding nekton utilization of tidal marshes, particularly within oligohaline and tidal freshwater environments. The succeeding chapters in this dissertation will address abundance and distribution patterns of sub-adult marsh-resident nekton in both tidal freshwater and salt marsh environments. In Chapter II, short-term abundance patterns of marsh-surface nekton are documented at polyhaline mainland and back-barrier marshes of the Virginia Coast Reserve (VCR). In Chapter III, abundance and distribution patterns of F. heteroclitus YOY at regularly and intermittently flooded mainland marshes of the VCR are described, along with patterns of spawning site utilization by this species. In Chapter IV, faunal composition, relative abundance and size/age class distribution are objectively compared within and between tidal freshwater and polyhaline salt marshes with varying flooding characteristics. Chapter V is a review of 10 years of nekton community ecology and habitat utilization research in tidal freshwater wetlands of the Chickahominy River, Virginia, with an evaluation of sampling methods. The distribution, composition and seasonality of meiofauna and the potential importance of meiofauna as prey for larval and juvenile fishes in tidal freshwater wetlands is examined in Chapter VI. This information should prove invaluable in the determination of the functional ecology of tidal freshwater and polyhaline salt marshes. A detailed understanding of the processes controlling distribution and abundance of resident finfish and invertebrates, and the identification of intermediate-level trophic relationships will enhance future efforts to manage existing natural marsh areas and ensure the effectiveness of marsh restoration efforts.
I compared mid-summer abundance of resident sub-adult finfish (Fundulus spp.) and daggerblade grass shrimp (Palaemonetes pugio) at mainland and back-barrier salt marshes. Pit traps were used to collect marsh surface nekton from June 10 - August 15, 1991 at two marshes located within the Virginia Coast Reserve Long-Term Ecological Research Site. Significantly greater abundance of fishes (but not grass shrimp) was observed at the mainland marsh. Bi-weekly periodicity in shrimp and finfish abundance was observed at the mainland site only. Site-specific and temporal patterns of sub-adult nekton abundance were determined primarily by differences in elevation and hydroperiod of each marsh. Spotfin killifish (Fundulus luciae), previously considered rare on Virginia's Eastern Shore, were frequently collected at the mainland marsh.
In summer 1991, I conducted a 10 week pilot study in order to evaluate the effectiveness of pit traps (Kneib, 1978; 1984; Talbot and Able, 1984) as a technique for estimating relative abundance of marsh-surface nekton (primarily juvenile cyprinodont fishes and decapods) on the surface of coastal salt marshes within the Virginia Coast Reserve barrier island-lagoon complex. Previous studies of marsh dependent/resident nekton populations at the VCR are few, and mostly limited to seine and trawl surveys scattered widely in space and time (Richards and Castagna, 1970; Norcross and Hata, 1990).
The importance of the vegetated marsh surface as habitat for larval and juvenile marsh-resident finfish and invertebrates has been emphasized (Boesch and Turner, 1984; Zimmerman and Minello, 1984; Kneib, 1984; 1986; 1987a; 1987b). Larval and juvenile fishes and decapods may forage effectively on the flooded marsh surface, yet avoid predation by seeking temporary refuge in shallow intertidal pools and rivulets at low tide. (Kneib, 1984; 1986; 1987a). Few previous studies have compared use of the marsh surface by resident sub-adult nekton at disparate marsh sites within a single dynamic system such as the Virginia Coast Reserve (VCR).
This study was conducted in marshes of the Virginia Coast Reserve Long-Term-Ecological Research Site (VCR-LTER). Within the VCR complex, salt marsh development occurs primarily as fringing coastal marshes associated with the mainland Delmarva Peninsula and as back-barrier marshes located on the landward side of barrier islands. Additional isolated marsh islands occur in mid-lagoon areas, however, they represent a relatively minor percentage of total marsh area in this system. Mainland marshes are accreting due to relative sea-level rise (subsidence) whereas back-barrier marshes are undergoing erosion (Hayden et al., 1991). A mainland salt marsh located in Northampton County, Virginia (USGS Nassawadox quadrangle) and a back-barrier marsh located on the northern end of Hog Island (USGS Quinby Inlet Quadrangle) were chosen for comparison (Figure 2.1). The mainland marsh was located along a second order tributary of Phillips Creek (hereafter referred to as Phillips Creek Marsh). Emergent vegetation at this site consisted primarily of medium to short form Spartina alterniflora with Salicornia virginica and Distichlis spicatum occurring throughout an extensive upper intertidal zone. This site was flooded entirely only on spring and storm tides and average depth of flooding in the lower intertidal zone was ~ 10 - 15 cm. The topographic profile of this marsh was relatively level, with a distinct berm (levee) adjacent to the creekbank. Flooding water (and presumably marsh-dependent nekton) accessed the marsh via several intertidal rivulets located along the creekbank, dissecting the berm.
The back-barrier marsh chosen for this study was a marsh island located at the northwest end of Hog Island. A tidal creek (Cattleshed Creek) flowed around the entire marsh, and a berm was present around the marsh perimeter. The tall form of Spartina alterniflora was the predominant vegetation present. Flooding water and nekton reached the marsh surface via a tributary creek entering at the western boundary of the marsh. The marsh was regularly flooded to depths of > 0.5 m.
I installed 10 clay pots (18 cm diameter, 18 cm depth) at randomly selected locations within the low marsh at each site. These traps emulated the shallow intertidal microhabitats available to sub-adult nekton at low tide (Kneib, 1984) and collected resident fishes and decapods remaining on the marsh as the tide receded. Traps were sampled weekly for a total of 10 visits to each site from June 10 through August 15, 1991. I attempted to sample on mornings of consecutive days during each week, however, some samples from both sites were collected during mid-day, as dictated by tidal conditions. Larval and juvenile fishes and grass shrimp were removed from traps by repeated circular sweeps with a small dip net. Initially, traps were left uncovered between sampling intervals. However, within several days, fiddler crab (Uca pugnax) carcasses filled the traps. Installation of nylon mesh covers (12 mm diam.) during the second week of the study significantly reduced the accumulation of crabs in traps and did not appear to inhibit use of the traps by sub-adult finfish and decapods. On each sampling date, I measured surface water temperature, salinity and dissolved oxygen content within traps using a stem thermometer, a Reichert-Jung temperature-compensated refractometer, and a YSI Model 57 Oxygen Meter. In addition, average stem density of emergent macrophytes was determined once in late July by counting individual plant stems in triplicate 0.0625 m2 plots located randomly at each site. In the laboratory, larval and juvenile fishes and shrimp were identified to species, counted and measured (TL, in mm). Total (unpreserved) biomass of organisms (mg wet wt.) per trap was determined. All specimens were fixed in 10% buffered formalin. Following fixation, all specimens were preserved in 70% ethanol and archived at the University of Virginia's Long-Term Ecological Research Laboratory in Oyster, Virginia.
Statistical analyses. I tested for differences in abundance of fishes and grass shrimp between each site using a repeated measures analysis of variance with SITE as the between subjects factor and SAMPLING DATE as the within subjects factor. Abundance data were normalized using a log (y + 1) transformation (Sokal and Rohlf, 1981). Statistical analyses were performed using SuperANOVA software for the Macintosh PC (Abacus Concepts, 1989).
Site Conditions. Artificial microhabitats at the mainland marsh were characterized by higher average salinity and temperature relative to back-barrier marsh microhabitats (Table 2.1). Mean dissolved oxygen content within traps was also higher at the mainland marsh. Hypersalinity in surface waters (66 ppt) was measured on July 16 at the mainland marsh. Maximum air temperature on this day was 30.0deg. C. Average monthly precipitation (Painter, Virginia Climate Station) was 75.1 mm, a -29.5 mm departure from the monthly normal (1951-1980). The average daily temperature at this station was 27.0deg. C., a 3.8deg. departure above the 1951-1980 station normal for July. (NOAA, 1991). Mean stem density of emergent macrophytes (primarily Spartina alterniflora) was nearly twice as high at the mainland marsh (mean = 502.4+41.1 m-2 vs. 249.9+13.0 m-2).
Abundance and Composition. I collected a total of 546 fishes and 1182 decapods during the 10 week study interval. Mummichogs (Fundulus heteroclitus) represented 94% of all fishes collected. Spotfin killifish (Fundulus luciae) comprised the remaining 6%. The daggerblade grass shrimp Palaemonetes pugio was the sole decapod species captured (exclusive of fiddler crabs Uca pugnax which were not considered nekton). Significantly more fishes were collected at the mainland marsh site (p = 0.0009). Grass shrimp abundance did not differ significantly between marshes (Table 2.2). There were significant SITE x SAMPLING DATE interactions for both taxa (p = 0.0001) Fundulus spp. occurred at both sites on all sampling dates. Grass shrimp were not collected until week 5 (early July). The most striking difference in abundance between the two sites is the occurance of a distinct bi-weekly periodicity in shrimp abundance at the mainland marsh (Figure 2.2). This trend is absent at the island marsh. A similar, though less extreme, pattern is observed for fishes at the mainland marsh.
Larvae and juveniles comprised 95% of total fishes collected. Larvae and juveniles comprised 68% of total grass shrimp collected. Greater proportions of total catch were represented by sub-adult fishes and shrimps at the mainland marsh (Table 2.3). A slightly greater range of sizes was also noted at the mainland marsh for both fishes and shrimps. Mean total length (mm) was greater for all three species at the back-barrier marsh.
The results presented here are consistent with previous studies of habitat use by sub-adult nekton in Atlantic Coast salt marshes. I observed an early summer abundance peak of cyprinodont (primarily Fundulus heteroclitus) larvae and juveniles in mainland marshes. A second peak, representing a later cohort, occurred in late summer. I did not sample into autumn, however, more recent data from mainland marsh sites indicates that an additional cohort is produced in September - October. These data are similar to patterns of abundance reported by Kneib (1986) for F. heteroclitus at Sapelo Island, Georgia. He documented three distinct abundance peaks corresponding to full moon phases of the lunar cycle. Abundance data for Palaemonetes pugio also correspond to observations of this species in other coastal systems. I observed a distinct bi-weekly periodicity in abundance of P. pugio from early July, when young individuals recruited to the marsh, to mid-August. Kneib (1987b) reported a similar bi-weekly periodicity in abundance pulses of sub-adult P. pugio in Sapelo Island marshes. Curiously, bi-weekly periodicities were absent in back-barrier marsh populations of fishes and shrimp in my study. The major difference between the two sites in this study was hydroperiod. My observations indicate that the back-barrier marsh flooded regularly and to a significant depth (~ 50 cm). In contrast, the mainland marsh was generally flooded only on spring tides and average flooding depth was relatively low (~ 10-15 cm). This marsh was subsequently instrumented with a Qualimetrics Richards-Type water level recorder. Recent data (March 1993 - present) confirm my earlier observations on flooding frequency/depth at this location.
Kneib (1993) reported that growth of F. heteroclitus larvae was positively associated with flooding duration, and hypothesized that tidal flooding controlled the renewal rate of prey resources available to larvae. My size/age composition data support this, with larger individuals collected from the back-barrier marsh (Table 2.3), however, my sampling techniques were selective for larvae and juveniles, and likely excluded late juveniles and adults at both sites.
I sampled a single location (lower intertidal zone) at each marsh in my comparison. In an earlier study (Kneib, 1984), significant variation in abundance of larval and juvenile cyprinodonts (Fundulus heteroclitus and Fundulus luciae) was reported across an intertidal transect at a Sapelo Island, Georgia salt marsh, with greatest abundance occurring in the upper intertidal zone. Larvae and early juveniles were most abundant at higher elevations, whereas larger juveniles and adults dominated collections from the lower intertidal.
It has been documented that spawning activity (as indicated by egg counts and gonadosomatic indices) in Fundulus heteroclitus peaks in concert with spring tides in mid-Atlantic marshes (Taylor et al., 1979). Kneib (1987b) suggested that grass shrimp temporal abundance patterns may be similarly influenced by lunar cycles, either via synchrony of reproductive activity or as a function of increased access to the marsh surface due to higher spring tides. The greater availability of high marsh at the mainland site may explain the higher abundance of resident finfish at that location. The back-barrier site was almost entirely low marsh, except for a restricted area of short-form Spartina alterniflora located upon the berm. If the high marsh environment is preferentially utilized as a spawning site and nursery area by Fundulus heteroclitus and Fundulus luciae (Byrne, 1978; Kneib, 1984; Talbot and Able, 1984) recruitment of larval and juvenile cyprinodonts would have been enhanced at our mainland site. Grass shrimp do not utilize the intertidal marsh surface as a spawning site. However, post-larval grass shrimp (6 - 8 mm) recruit from sub-tidal creeks to the intertidal marsh surface during mid- to late summer (Kneib, 1987). At this time, grass shrimp are the numerically dominant organism present on the lower intertidal marsh surface.
My observations are not intended to suggest that all mainland marshes support greater abundance of marsh-resident nekton relative to all back-barrier locations at the VCR. Many back-barrier marshes at the VCR are contiguous with terrestrial island environments and contain substantial high marsh. However, marsh islands such as Cattleshed Creek Marsh are common at back-barrier locations. My intention was to illustrate variation in marsh types within the VCR and compare patterns of habitat utilization by resident sub-adult nekton between disparate marshes.
I collected 29 sub-adult and adult spotfin killifish (Fundulus luciae) at the mainland marsh site. Three adults were collected at the back-barrier marsh. Richards and Bailey (1967) concluded that this species is either rare or occupies a limited niche on the seaside of Virginia's Eastern Shore. Byrne (1978) reported on the life history of this species from the York River drainage, Virginia and suggested that populations of this species may have been previously overlooked in Virginia. Similarly, Able et al., (1983) and Shields and Hayes (1983) have reported spotfin killifish to be locally abundant in New Jersey and North Carolina high marshes, respectively. I have collected this species from high marsh shallows, ponds and ditches at the VCR-LTER on numerous occasions in 1991 - 1993 and concur with the previously mentioned studies that F. luciae is underepresented in conventional seine and trawl surveys of coastal marshes due to the dependence of this species on upper intertidal marsh habitats.
Resident sub-adult finfish (Fundulus spp.) were more abundant at a mainland salt marsh relative to a back-barrier marsh. Abundance of daggerblade grass shrimp (Palaemonetes pugio) did not significantly differ between marshes. Between-site differences in elevation and hydroperiod and the relative availability of high marsh habitat are potential factors influencing the observed patterns of abundance.
Spotfin killifish were frequently encountered in this study and are apparently not uncommon in high marsh habitats at the VCR-LTER. As suggested by previous investigators, the purported rarity of Fundulus luciae in mid-Atlantic salt marshes is due to under-representation by conventional sampling techniques combined with specific habitat requirements.
Abundance and spawning site utilization in a population of the common mummichog Fundulus heteroclitus were compared at regularly and irregularly-flooded mainland salt marshes at the Virginia Coast Reserve (VCR) from April - November 1992. Mummichogs are the numerically dominant fish species present in intertidal and shallow subtidal salt marsh environments on the U.S. east coast and are an important intermediate-level consumer in salt marsh trophodynamics Mummichog abundance was greatest in June. Mummichogs comprised 83% of all fishes collected on intertidal marsh surfaces. Significantly more mummichogs were collected at the regularly flooded marsh (ANOVA, p = 0.007). Young-of-the-year represented a greater proportion of total mummichogs collected (72%) at the regularly flooded marsh in comparison to the irregularly flooded marsh (61%). In general, mummichogs were more abundant in the lower intertidal zone relative to the upper intertidal. Mummichogs preferentially utilize empty ribbed mussel Guekensia demissa shells as egg deposition sites in Virginia Coast Reserve marshes; however, egg distribution is patchy, and patterns were not readily discerned. These results support the contentions of previous workers that a large-scale, intensive sampling effort is neccessary to accurately quantify spawning site utilization in salt marsh populations of F. heteroclitus.
The mummichog, Fundulus heteroclitus, is a ubiquitous component of salt marsh nekton communities along the Mid-Atlantic coast. Production of this species in mid-Atlantic salt marshes is among the highest reported for fishes (> 40.7 g m-2 year-1) and sub-adults may account for approximately 80% of total annual mummichog production (Merideth and Lotrich, 1979). Mummichogs represent a significant vector of energy flow into and out of tidal marsh ecosystems due to their high densities and widespread distribution (Vince et al, 1976). Mummichogs occupy both predator and prey positions in salt marsh food webs. Predation by F. heteroclitus may control the abundance and size distribution of marsh surface-dwelling invertebrates (Kneib, 1987). Marsh-dependent predators, including Summer flounder Paralichthys dentatus, juvenile bluefish Pomatomus saltatrix, and blue crabs Callinectes sapidus consume mummichogs in salt marshes (Kneib, 1982; Rountree and Able, 1992; This Volume, Appendix I).
In the mid-Atlantic region, F. heteroclitus spawns in conjunction with spring tides and deposits its dessication - resistant eggs in the empty shells of the ribbed mussel Guekensia demissa or attaches them to the base of Spartina alterniflora stems and leaves (Able, 1984; Taylor and DiMichele, 1983). Reproductive condition is highest for several days coincident with full or new moons (Taylor and Dimichele, 1980). This tidal synchrony ensures deposition of eggs in the upper intertidal zone, where they are least likely to be removed by tidal currents (Taylor et al.,1979). Egg hatching is triggered by appropriate conditions of submergence and low dissolved oxygen levels, but eggs may remain viable for up to 1 month exposed to air (Taylor et al., 1977; DiMichele and Taylor, 1980). Using in situ manipulations, Kneib (1993) demonstrated that growth rate was positively associated, and mortality rate negatively associated with tidal flooding for successive cohorts of F. heteroclitus larvae in a Sapelo Island, Georgia salt marsh.
In this study, abundance and spawning site utilization were investigated in a marsh population of Fundulus heteroclitus at the Virginia Coast Reserve Long-Term Ecological Research Site (VCR-LTER). The primary study objective was to document abundance and distribution patterns of F. heteroclitus (primarily young-of-the-year) on the surface of two mainland salt marshes varying in hydroperiod (regular vs. irregular flooding) within the VCR-LTER. A secondary objective was to quantify distribution and abundance of F. heteroclitus eggs on the surface of salt marshes within the VCR-LTER in order to determine if spawning site utilization within mainland marshes of the VCR-LTER was similar to that observed in other mid-Atlantic populations of F. heteroclitus.
Two salt marsh sites at the Virginia Coast Reserve were selected for study (Figure 3.1). The two marshes varied in surface topography and flooding regime. Site 1 was located along a 2nd order tributary of Phillips Creek. Vegetation type was typical of mid-Atlantic high marsh environments with Salicornia virginica and Distichlis spicata dominating from the forested upland boundary to the mid-marsh. From the mid-marsh to the creekbank, the short-form of Spartina alterniflora occured. Medium to tall S. alterniflora occured only in a narrow fringe surrounding intertidal rivulets at this site. Maximum flooding depth was generally < 10-15 cm and the upper marsh was flooded only during spring tides. The second site was located along a 1st order tributary of an unnamed tidal gut and was separated from Phillips Creek by a man-made causeway. This site was adjacent to a wooded area known locally as "The Hammocks" and is hereafter referred to as "Hammocks Marsh" This marsh was flooded regularly in excess of 30 cm depth. At this site, S. virginica and D. spicata were restricted to a narrow band adjacent to the upland boundary. Short-form S. alterniflora progressively graded to tall form in the mid-low marsh.
Pit traps (Kneib 1984; Talbot and Able 1984) were used to collect mummichogs and other marsh-resident nekton at four stations along elevational transects at Phillips Creek Marsh and Hammocks Marsh. Traps consisted of the bottom half of a 11.4 liter gallon plastic storage basin which was placed into a pit dug into the marsh substrate. A 0.9 x 1.2 m length of 1.6 mm nylon mesh netting was placed into the trap as a removable liner. Four 85 gm pyramid sinkers were attached to the net in order to conform the liner to the bottom of the trap. Two 1.2 m lengths of 1.9 cm diameter PVC pipe were attached lengthwise to the mesh liner and used as brails to purse the net when removing the sample.
Three replicate traps were installed at each topographic stratum along a transect at each site. Sampling was conducted monthly at maximum predicted spring low tides from April through November, 1992. Organisms collected in traps were placed on ice and returned to the laboratory for analysis. Unpreserved samples were sorted immediately following collection. All marsh-surface nekton (fishes and decapod crustaceans, excluding fiddler crabs (Uca pugnax) and marsh crabs (Sesarma reticulatum) were identified, counted, and preserved in 10% buffered formalin solution. For the purpose of this study, mummichogs were counted and measured to total length, in mm.
Differences in abundance of mummichogs between the two study sites and between sampling stations within sites were tested by a repeated measures analysis of variance (ANOVA) model with SITE and STATION as between subjects factors and SAMPLING DATE as a within subjects factor. Abundance data were normalized using a log (y + 1) transformation (Sokal and Rohlf, 1981). Post-hoc means comparisons (Student-Newman-Keuls test) and a priori paired contrasts were used to compare means when significant differences were specified ([[proportional]] = 0.05).
The relative contribution of two age classes (young-of-the-year, adult) was determined by generation of frequency tables. Age/size class assignments were based on literature reported sizes for F. heteroclitus (Kneib and Stiven 1978, 1987). Statistical analyses were performed using SuperANOVA and Statview II software for the Macintosh PC (Abacus Concepts, 1989).
Measurements of physico-chemical parameters (salinity, temperature, DO, pH) within marsh surface waters were taken on each sampling date at all sites using a temperature compensated refractometer, a stem thermometer, a Hanna portable pH meter, and a YSI Model 57 Oxygen meter.
Permanent 100 m longitudinal transects were established within each of the four elevational strata at all sites. Empty ribbed mussel (Geukensia demissa) shells were collected and Spartina alterniflora stems were harvested from randomly selected 1 m2 sample plots along each transect on June 4, June 15, July 2 and August 4 from Phillips Creek Marsh and Hammocks marsh. Six plots per transect were sampled on June 4 and June 15 and sampling effort was increased to 8 plots per transect for the latter two sampling dates due to the patchy distribution of empty shells. Additional samples were collected on June 5 from 100 m2 permanent plots located at the upper boundary of the low marsh at each site. In the laboratory, S. alterniflora stems and contents of mussel shells were carefully rinsed onto a # 60 (250 u m) brass soil sieve and examined for F. heteroclitus eggs. All eggs collected were preserved in 10% buffered formalin. Shell width (length of long axis, in mm) was measured for each empty shell (Taylor and DiMichele, 1983). Live mussels were censused from each sample plot in order to determine the relative availability of mussel shells as spawning sites at each topographic stratum. In addition, stem densities of emergent vegetation were measured along all transects on June 8.
Fundulus heteroclitus comprised 83% of all fishes collected at these sites during 1992. Additional marsh resident fish species collected in traps included spotfin killifish Fundulus luciae and naked goby Gobiosoma bosci. Daggerblade grass shrimp Palaemonetes pugio, juvenile blue crabs Callinectes sapidus and juvenile big-clawed snapping shrimp Alpheus heterochaelis were also frequently collected in pit traps.
A total of 634 mummichogs were collected during the study interval. In general, mean abundance was greatest at creekbank and low marsh stations, with decreasing abundance at high marsh and marsh/upland interface stations (Figure 3.2). Abundance peaked in June, when large numbers of post-larvae and early juveniles were present on the marsh surface. Overall, YOY comprised 72% of all mummichogs collected at Hammocks Marsh, and 61% of mummichogs collected at Phillips Creek Marsh. Significantly more mummichogs (66% of total) were collected at Hammocks Marsh than at Phillips Creek Marsh (ANOVA, p = 0.007), many of which (37% of total) were collected from the low marsh station. At Hammocks Marsh, significant differences were observed between the marsh/upland interface, where relatively few fishes were collected, and all other stations (p < 0.005). At Phillips Creek Marsh, significant differences in abundance were observed between the marsh/upland boundary and the high marsh (p = 0.0006) and creekbank (p = 0.0015) stations. Relatively few mummichogs were collected in the low marsh at Phillips Creek. Abundance was significantly greater at the high marsh station relative to the low marsh (p = 0.0197) at this site. Recruitment to the marsh surface and/or spawning activity appears to occur earlier at Hammocks Marsh than at Phillips Creek Marsh, as indicated by greater abundance of YOY in April - June. After July, however, mean monthly abundance was slightly greater at Phillips Creek Marsh. The major difference between the two sites in our study was variation in hydroperiod. Our observations indicate that Hammocks Marsh flooded regularly and to a significant depth (~ 30 cm). In contrast, Phillips Creek Marsh was generally flooded only on spring tides and average flooding depth was relatively low (~ 10 -15 cm). The two marshes was subsequently instrumented with a Qualimetrics Richards-Type water level recorders. Recent data (March 1993 - present) confirm our earlier observations on flooding frequency/depth at these locations. Kneib (1993) experimentally determined that growth rate was positively correlated with flooding duration in a Sapelo Island, Georgia population of F. heteroclitus. Our abundance and size-distribution data indicate greater recruitment and survivorship at Hammocks Marsh, a regularly flooded site.
A total of 1706 Fundulus heteroclitus eggs were collected from transects and 100 m2 mid-marsh plots; however, these numbers are insufficient to accurately assess distribution and abundance patterns (Table 3.2). Although eggs were collected at both sites, abundance was extremely patchy, and often a single shell would yield large numbers of eggs, indicating multiple spawns (Taylor and DiMichele, 1983).
Live mussels were most abundant at the low marsh and creekbank transects at Phillips Creek. At the Hammocks, mussels were most abundant in the high marsh and low marsh zones. In general, few empty shells were found in transect plots (n = 48). All transects yielded empty shells except for the upland boundary at Phillips Creek. Eggs were found in shells at the creekbank zone at Hammocks marsh and in the low marsh at Phillips Creek where a single shell contained 657 eggs.
Very few eggs were found attached to Spartina alterniflora stems in the intertidal zone; four were collected at the marsh/upland interface at Phillips Creek, where G. demissa does not occur. Mean stem density was highest at this location (Table 3.3). A single egg was collected in the low marsh transect at Hammocks Marsh.
Collections at the Hammocks Marsh 100 m2 plot on June 5 yielded 29 empty shells; only 4 empty shells were recovered from the Phillips Creek Marsh plot (Table 3.5). Shell width at the Phillips Creek Marsh plot ranged from 75 - 102 mm (mean = 90.0 +5.5). Shell width ranged from 57 - 106 (mean = 89.0 +1.8) at Hammocks Marsh (Table 3.4). A total of 1031 eggs were found in empty shells from the Hammocks Marsh plot. Number of eggs per shell ranged from 0 - 613 (mean = 35.5 +22.8). No eggs were found in the shells from the Phillips Creek Marsh 100 m2 plot.
Previous investigators (Able and Castagna, 1975; Taylor and DiMichele, 1983) have reported that selection of suitable shells for egg deposition depends on orientation and gape width; eggs were deposited only in shells oriented vertically with a gape width of 0.5 - 5 mm. Although shells were not examined for gape width in our study, this variable may account for the lack of eggs in otherwise suitable shells. These authors also reported that egg deposition was likely to occur only in shells > 60 mm in width. Shells < 50 mm were never utilized as egg deposition sites in Delaware marshes or in our Virginia mainland marshes.
Results presented here are similar to those reported by Taylor and DiMichele (1983) in their investigation of spawning site utilization by F. heteroclitus in a Delaware salt marsh. They surmised that S.alterniflora stems were probably used as a secondary spawning site by F. heteroclitus, and that egg deposition on S. alterniflora stems and leaves was likely to be of greater significance in low salinity coastal marshes where G. demissa did not occur. In a related study, we have observed widespread deposition of eggs at the base of Arrow-arum (Peltandra virginica) stems in a tidal freshwater population of F. heteroclitus residing in marshes contiguous with the Chickahominy River, Virginia. Occasional additional collections from back-barrier salt marshes at the Virginia Coast Reserve have yielded extremely high densities (> 2500 eggs per shell). These observations suggest a greater degree of Guekensia shell utilization at back-barrier marsh sites relative to mainland sites. Intensive comparative collection efforts at back-barrier and mainland marshes are recommended for future study.
Mummichogs were significantly more abundant at The Hammocks marsh site, where regular tidal flooding may enhance survivorship and growth of marsh-resident nekton. In general, relative abundance of mummichogs (primarily YOY) was greater at creekbank and low marsh stations relative to the upper intertidal.
Mummichogs preferentially utilize the empty shells of the ribbed mussel Geukensia demissa, which are abundant and widely distributed on the surface of VCR salt marshes, as egg deposition sites. However, as documented elsewhere, occurrence of eggs on the marsh surface is patchy, and patterns of distribution and abundance are not readily discerned.
The results of this study concur with those of previous workers (Taylor and DiMichele, 1983) and support their recommendation that a large-scale, intensive sampling program would be neccessary to accurately identify patterns of egg deposition by F. heteroclitus on the surface of salt marshes.
Habitat-specific patterns of abundance and distribution of sub-adult marsh surface nekton were investigated at tidal freshwater and salt marsh sites in Virginia. Intertidal marsh nekton are important vectors of detrital energy transfer in coastal marsh systems. This function has been well-documented for salt marshes on the US. East Coast; however, few previous studies have documented nekton composition and abundance/distribution patterns for intertidal freshwater ecosystems. Pit traps were used to collect nekton along elevational transects at four sites representing variation in surface hydroperiod from April through November 1992 - 1993. The dominant fish collected at all sites was the mummichog Fundulus heteroclitus. The grass shrimp Palaemonetes pugio was the dominant species collected at salt marsh sites, and was seasonally abundant on tidal freshwater marshes. A positive correlation between marsh hydroperiod and nekton abundance/biomass was observed at salt marsh sites; an opposite pattern was observed at tidal freshwater marshes. Tidal flooding regime influences the abundance of resident nekton; however, the effect may be confounded by other environmental variables, including variation in surface micro-topography and the seasonal presence or absence of submerged aquatic vegetation (SAV) in the adjacent sub-tidal zone. In tidal freshwater, SAV provides a predation refuge and food source for early life stages of marsh-dependent nekton, and several species may utilize this environment extensively. Salt marshes, in contrast, lack SAV in the sub-tidal zone. Consequently, between-site differences in species and size-specific marsh surface utilization by resident nekton were observed. Larvae and juveniles represented 79% and 59% of total fishes collected at tidal freshwater and salt marsh sites, respectively. Despite physico-chemical differences and variation in general community composition between tidal freshwater and salt marshes, the resident sub-adult nekton community of disparate tidal marsh surfaces is similar, characterized by a few ubiquitous species with broad environmental tolerances.
Utilization of the tidal marsh environment by estuarine-dependent nekton is a well-recognized, yet incompletely understood function of tidal wetlands. Several authors have documented the use of coastal salt marshes as a food source and predation refuge for larvae and juveniles of estuarine and marine species (Shenker and Dean, 1979; Weinstein, 1979; Kneib, 1984; Talbot and Able, 1984). Although the habitat value and nekton community structure of tidal freshwater marshes has recently been explored (Rozas and Odum, 1987a, 1987b, 1987c, 1988; McIvor and Odum, 1988), utilization of tidal freshwater wetlands specifically by sub-adult nekton remains poorly documented.
Assessing the habitat value of a marsh depends in part on use of the marsh surface by resident fishes. Marsh resident species have been previously defined as those which complete their entire life cycle within the marsh (Hettler, 1989). As low tide approaches and the marsh surface drains, resident nekton must either move off the marsh and into adjacent subtidal habitats where they may be subjected to increased predation pressure (McIvor and Odum, 1988) or take refuge in shallow microhabitats on the marsh surface (Kneib, 1984).
The primary limitation to adequate documentation of habitat use by sub-adult nekton in tidal marshes is the difficulty in quantitatively sampling the vegetated marsh surface. Several recent studies have addressed this problem (Zimmerman and Minello, 1984; Kneib, 1984; 1991; McIvor and Odum, 1986 ; Rozas, 1993). Unfortunately, due to the wide variety of sampling gear employed, the results of most contemporary studies cannot be compared effectively . In this study, I sought to ameliorate this problem by introducing a standardized sampling protocol for comparison of sub-adult nekton habitat utilization and community structure on tidal freshwater and coastal salt marsh surfaces.
The objective of this study was to compare patterns of abundance, distribution, and habitat utilization by sub-adult nekton within and between tidal freshwater and salt marshes. The role of three environmental variables (marsh topography, flooding regime, and geographic location along a salinity gradient) in determining the nursery function of the tidal marsh surface were examined (Fig. 4.1).
Preliminary observations indicated that the average frequency, size and depth of intertidal pools in tidal freshwater marshes increased along an elevation gradient from upper to lower intertidal locations. Consequently, I hypothesized that resident fishes would be more abundant at low marsh sites than at high marsh locations. The second variable for comparison was marsh hydroperiod. I hypothesized that resident fishes would be more abundant in a marsh with greater average inundation time and depth of flooding, due to an increase in available foraging or predator avoidance time and a reduction in potential dessication and/or heat stress. The third variable to be investigated was location along a salinity gradient. I sought to compare general patterns of habitat use and community composition of resident nekton within and between tidal freshwater marshes and salt marshes. A direct, standardized comparison of the two habitat types is unprecedented.
A fundamental difference between tidal freshwater and salt marshes is the seasonal presence or absence of submerged aquatic vegetation (SAV) in shallow subtidal areas. Submerged vegetation provides high-quality forage habitat and a predation refuge for a variety of freshwater and estuarine-dependent nekton species in tidal freshwater. Several species may utilize this environment extensively, in addition to the intertidal marsh surface, when available (Rozas and Odum, 1987b). In contrast, salt marshes lack SAV in the shallow sub-tidal zone. This habitat disparity may influence foraging patterns and predation risk in salt marsh creeks. Consequently SAV presence or absence may affect the relative abundance, size and age class structure and species composition of resident marsh surface nekton.
The primary study site was located within the Chickahominy Wildlife Management Area, in Charles City County, Virginia. This site is contiguous with the Chickahominy River, a tributary of the James River sub-estuary of the Chesapeake Bay (Fig. 4.2). Mean tidal amplitude in this area is 0.7 m and mean salinity is generally < 0.5 ppt, except under extreme drought conditions (McIvor and Odum, 1988) Two marshes were selected to represent variation in hydroperiod. Eagle Bottom Marsh represented a fringing tidal freshwater marsh in a relatively early stage of development. The majority of the marsh was dominated by a single emergent plant species, Peltandra virginica. A narrow fringe of wild rice, Zizania aquatica, and giant cutgrass, Zizaniopsis mileacea occured in the high marsh, adjacent to a steep hillslope. Previous studies at this site have focused on nutrient cycling and groundwater hydrology (Chambers and Odum, 1990; Harvey and Odum, 1990). A perched marsh contiguous with Beaver Dam Creek represented a later developmental stage, characterized by a distinct raised creekbank and vegetation patterns resulting from a shorter hydroperiod compared to Eagle Bottom Marsh. This high marsh is dominated by wild rice (Zizania aquatica) with a relatively narrow zone of P. virginica occurring at the creekbank or along intertidal rivulets early in the growing season. Southern wild rice (Zizaniopsis mileacea) is abundant along the marsh/upland boundary.
Salt marsh sites were located at the Virginia Coast Reserve Long-Term Ecological Research site (VCR-LTER) near Nassawaddox, Virginia (Fig. 4.2). Two marshes were selected to represent variation in hydroperiod similar to the comparison outlined for the tidal freshwater marsh sites. Marsh 1 was located along a 2nd order tributary of Phillips Creek and represented a typical high marsh (infrequently flooded). Vegetation type was typical of mid-Atlantic high marsh environments with Salicornia virginica and Distichlis spicata dominating from the forested upland boundary to the mid-marsh. From the mid-marsh to the creekbank, the short-form of Spartina alterniflora occured. Medium to tall S. alterniflora occured only in a narrow fringe surrounding intertidal rivulets at this site. Maximum flooding depth in the low marsh at this site was generally < 10 cm and the upper marsh flooded only during spring tides. The second salt marsh site was located along a 1st order tributary of an unnamed tidal creek and was separated from Phillips Creek Marsh by a man-made causeway. This site was adjacent to a wooded area known locally as "The Hammocks" and is hereafter referred to as "Hammocks Marsh". This marsh was flooded regularly in excess of 30 cm depth. At this site, S. virginica and D. spicata were restricted to a narrow band adjacent to the upland boundary. Short-form S. alterniflora progressively graded to tall form in the mid-low marsh. Mean tidal amplitude at the VCR-LTER is 1.3 meters.
At all sites, transects were established and pit traps were used to sample larval and juvenile fish from distinct topographic strata upon the marsh surface. Pit traps have been used previously in salt marshes (Kneib and Stiven, 1978; Kneib, 1984; Talbot and Able, 1984) and rely on the dependence of larval and juvenile nekton on tidal flooding of the marsh surface. Nekton utilize natural depressions or pits in the marsh surface as refuge as the marsh drains, rather than move off of the marsh into adjacent creeks, where they would be subject to predation. Pit traps simulate these natural depressions and provide nekton with an artificial refuge.
Traps consisted of the bottom half of a 11.4 liter plastic storage basin which was placed into a pit dug into the marsh substrate. A 0.9 x 1.2 m length of 1.6 mm nylon mesh netting was placed into the trap for use as a removable liner. Four 85 gm pyramid sinkers were attached to the net in order to conform the liner to the bottom of the trap. Two 1.2 m lengths of 1.9 cm diameter PVC pipe were attached lengthwise to the mesh liner and used as brails to purse the net when removing the sample. Three replicate traps were installed at each topographic stratum (marsh-upland interface, high marsh, low marsh, creekbank) along a transect at each site. Sampling was conducted monthly at maximum predicted spring low tides from April through November, 1992 and 1993. Organisms collected in traps were placed on ice and returned to the laboratory for analysis. Nekton were identified, counted and weighed. Fishes and decapods were measured to the nearest mm (total length for fishes, carapace width for crabs, tip of rostrum to end of telson for shrimp). Differences in abundance and biomass of fishes and numerically dominant decapod crustaceans (grass shrimp) between the two study sites and between sampling stations within sites were tested with a repeated measures analysis of variance (ANOVA) model with MARSH and ELEV STRATA as between subjects factors and SAMPLING DATE as a within subjects factor. Abundance and biomass data were log-transformed prior to analysis (Sokal and Rohlf, 1981). Post-hoc means comparisons (Student-Newman-Keuls test) and a priori paired contrasts were used to compare means when significant differences were specified ([[proportional]] = 0.05). Data from April 1993 were omitted from statistical analyses due to a severe coastal storm which occurred immediately prior to the collection date for salt marsh sites and may have biased the results against comparison to tidal freshwater sites, which were not affected by the storm. Many adult mummichogs Fundulus heteroclitus were stranded on the intertidal marsh surface at this time, presumably as a result of unusually high tides associated with the storm. The relative contribution of two age classes (sub-adult, adult) was determined for all fishes and numerically dominant decapod crustaceans (grass shrimp) by generation of frequency tables. Age/size class assignments were based on literature reported sizes for each species (Kneib, 1978; 1987; Kneib and Stiven, 1978; Lee et al., 1980). Statistical analyses were performed using SuperANOVA and Statview II software for the Macintosh PC (Abacus Concepts, 1989).
Measurements of physico-chemical parameters (salinity, temperature, DO, pH) were taken on each sampling date at all sites using a temperature compensated refractometer, a stem thermometer, a Hanna portable pH meter, and a YSI Model 57 Oxygen meter. Beginning in April 1993, all marsh sites were instrumented with Qualimetrics Richards-type water level recorders for determination of tidal flooding frequency, and average duration and depth of tidal flooding.
Physico-chemical parameters are summarized in Table 4.1. The most obvious difference between years is measured salinity at the tidal freshwater marsh sites. Unusually low rainfall during 1993 led to significant penetration of the salt front up into the tidal freshwater Chickahominy River. Measurable salinity (1 - 5 ppt) was observed at the Chickahominy River sites from August - November 1993. Marsh flooding characteristics (depth, duration of flooding) are summarized for each site in Table 4.2.
A total of 3271 fishes and 10042 decapod crustaceans (1998.46 gms. wet wt.) representing 12 estuarine dependent and/or freshwater species were collected in traps throughout the 2-year study period (Table 4.3). Species composition between freshwater and salt marsh sites was similar, with mummichogs Fundulus heteroclitus and grass shrimp Palaemonetes pugio numerically dominating collections at both sites (Table 4.4). Two additional ubiquitous marsh species, the blue crab Callinectes sapidus and the naked goby Gobiosoma bosci occured consistently, though not abundantly, at both sites. The mosquitofish Gambusia affinis holbrooki was the second most abundant fish species collected in tidal freshwater. The spotfin killifish Fundulus luciae, was the second most abundant fish species collected at salt marsh sites. Markedly greater abundance of both G. affinis and F. luciae was observed in 1993 collections relative to those from 1992. Additional fish species (collected infrequently) included banded killifish Fundulus diaphanus and brown bullhead Ameiurus nebulosus at tidal freshwater marshes and striped killifish Fundulus majalis at salt marsh sites.
Additional vertebrates collected on tidal freshwater marshes included green frogs Rana clamitans and a single spotted turtle Clemmys guttata collected at Beaver Dam Creek in July 1992. Northern water snakes Nerodia sipedon were commonly observed on tidal freshwater marsh surfaces, although none were collected in traps.
Total abundance of fishes increased sharply in mid-summer (July) at Beaver Dam Creek (tidal freshwater marsh; short hydroperiod). Very few fishes were collected at Eagle Bottom marsh (tidal freshwater marsh; long hydroperiod) during either year. At salt marshes, significantly greater abundance of fishes (ANOVA, p = 0.0001) was observed at Hammocks Marsh (long hydroperiod). In general, fish abundance was greater at salt marsh sites in 1993 relative to 1992 (Fig. 4.3). Fish abundance at freshwater marsh sites was virtually identical between the two sampling years (Fig. 4.4). At salt marshes, fish abundance was highest in June - July. At freshwater sites, fish were most abundant during July-August. Total fish biomass was greatest at the Phillips Creek salt marsh site, overall (Figs. 5 and 6). Overall, relatively few fishes were collected at the uppermost topographic level (marsh/upland interface) at all sites. However, this is the preferred habitat for juvenile and adult spotfin killifish Fundulus luciae (Weisberg, 1986), and F. luciae were often collected at this stratum at both salt marsh sites. A significant difference in abundance was observed between the marsh-upland interface and all other topographic strata at all sites (ANOVA, p < 0.05), except for Eagle Bottom Marsh, where very few fish were collected. Patterns of fish abundance/biomass were generally similar among the other three topographic levels with highest mean abundance/biomass occuring in the low marsh strata at all sites during the 2-year study interval (Tables 4.5 - 4.7, 4.9).
The majority of decapods collected at all marsh sites were grass shrimp Palaemonetes pugio. At salt marshes, adult P. pugio were present in moderate numbers in April and May. Very few shrimp were collected in June; however, in July and August, numerous late juvenile and early adult shrimp had been recruited to the marsh surface at both salt marsh sites, where they remained abundant through November. In general, shrimp abundance at the salt marsh sites was highest in the low marsh areas at both sites during July - August 1992 and August - September 1993; however, numerous shrimp were also collected in the high marsh and at the marsh/upland interface at Hammocks Marsh (salt marsh; long hydroperiod) in August 1992 and 1993 (Figs. 7 - 10; Tables 4.5, 8, and 10). Significantly greater abundance and biomass of P. pugio was collected from the Hammocks marsh site, overall (ANOVA, p = 0.0001).
Numerous grass shrimp emigrated to lower intertidal freshwater marsh surfaces during September - November of both years. Grass shrimp abundance in 1993 was nearly an order of magnitude greater than that of 1992. The occurence of grass shrimp on the marsh surface was coincident with the rapid senescense of submerged aquatic vegetation (Najas minor and Ceratophyllum demersum) in the adjacent subtidal. Grass shrimp are the numerically dominant crustacean taxa present in grass beds throughout the summer (see Appendix VI). Only when this habitat becomes unavailable do grass shrimp select the intertidal marsh surface as refuge habitat. Juvenile blue crabs (Callinectes sapidus) were occasionally collected at tidal freshwater salt marsh sites in April and May, 1992 and 1993, and many small juveniles were collected at the salt marsh sites in August. Juvenile snapping shrimp (Alpheus heterochaelis) were occasionally collected from the low marsh surface at both salt marsh sites from July - September. Juvenile crayfish (Cambarus robustus) were collected at both tidal freshwater marsh sites, primarily at the marsh/upland interface and high marsh strata.
The white-fingered mud crab Rhithropanopeus harrisi was collected August - November 1993 from tidal freshwater marshes but was not collected in 1992. Like P. pugio, this species may be abundant among submerged vegetation of tidal creeks (Rozas and Odum, 1987a) and apparently migrates to the lower intertidal marsh surface when SAV habitat is not available. Additional invertebrates present in traps at tidal freshwater sites included the amphipod Gammaraus fasciatus and the isopod Caecidotea spp., the gastropod Physa integra, leeches (Hirudinea) and dragonfly larvae (Odonata). The brackish-water fiddler crab Uca minax is present on Chickahominy River marshes, but is either uncommon or secretive at this locale, as none were collected in traps. Burrowing activity was rare and restricted to erosional creekbank locations. In contrast, the mud fiddler crab Uca pugnax and the marsh crab Sesarma reticulatum were frequently observed in salt marsh traps. The mesh liner permitted many semi-terrestrial crabs (and perhaps many blue crabs) to crawl out of traps. Consequently, blue crab abundance on the marsh surface at all sites is probably severely underestimated using pit traps. Similarly, crayfish abundance at tidal freshwater locations may also be underestimated, since they were also able to crawl out of traps. Additional invertebrates observed in salt marsh traps included the polychaete Nereis succinea, amphipods (Gammaridae) and the gastropod Melampus bidentatus. The mud dog whelk Nassarius obsoletus was abundant in traps located at the creekbank and low marsh strata at Hammocks marsh (salt marsh; long hydroperiod). These gastropods were rarely observed at locations in the higher intertidal.
A marked contrast in age class composition of marsh-resident fishes was observed between tidal freshwater and salt marsh sites. In tidal freshwater, > 80% of fishes collected were young-of-year. In salt marshes, YOY represented < 60% of total fishes collected (Fig. 4.11). All age classes of grass shrimp were present at both tidal freshwater and salt marsh sites, with juveniles predominant in salt marsh collections in July and August. Most grass shrimp collected on tidal freshwater marsh surfaces were adults. Blue crabs, and snapping shrimp from salt marsh sites were all sub-adults. Blue crabs and crayfish from tidal freshwater sites were all sub-adults; mud crabs collected were primarily adults.
Previous studies on utilization of intertidal marsh habitats have focused on use of the marsh surface by marsh-dependent nekton during the period in which the marsh remains flooded. In this study, emphasis was on that component of the nekton community which resides upon the marsh surface during both flooded and non-flooded conditions. Consequently, the total number of species reported here is relatively low, as few nekton species are adapted to withstand the environmental stresses inherent to the marsh surface, including dessication, salinity and heat stress. Larger individuals which may forage upon the marsh surface at flood tide are obviously excluded, due to the shallow depth and relatively small size of intertidal refuges. McIvor and Odum (1988) collected 28 species from intertidal marsh surfaces contiguous with Morris Creek, a Chickahominy River tributary. Rozas and Odum (1987c) also reported that 28 species utilized the marsh surface at Parsons Island marsh, located approximately 0.3 km upriver from the Beaver Dam Creek and Eagle Bottom Marsh study sites. In both of these studies, nekton were collected from the flooded marsh surface using flume nets. This gear type effectively samples a wide range of species and size classes; however, it may integrate nekton collected upon the flooded marsh surface with those collected at the marsh edge/subtidal ecotone, where nekton may be locally abundant and diverse (Baltz et al., 1993). Hettler (1989) used block nets to sample nekton leaving flooded marsh surfaces at Beaufort, North Carolina. He reported 35 fish species and at least 5 crustacean species as using the flooded marsh surface. Similarly, these collections include species which may only use the flooded intertidal for a limited time period or which may be concentrated in the vicinity of the marsh edge/subtidal ecotone. Kneib (1984) used pit traps to sample larval and juvenile fishes from the surface of intertidal salt marshes at Sapelo Island, Georgia. Two cyprinodonts (Fundulus heteroclitus and Fundulus luciae) accounted for > 96% of total fishes collected. Similarly, Talbot and Able (1984) reported four species of cyprinodonts (F. heteroclitus, F. luciae, Cyprinodon variegatus, and Lucania parva) as occupying high marsh surface habitats in New Jersey.
Nekton abundance and biomass were positively correlated with flooding duration and depth, as hypothesized, in salt marshes. In tidal freshwater marshes, a different pattern was observed. Abundance of nekton at Beaver Dam Creek was generally comparable to that of the salt mash site. The Eagle Bottom Marsh intertidal habitat was virtually unused; however, large schools of fishes (primarily Fundulus spp. and Gambusia affinis holbrooki) have been observed to forage upon the flooded intertidal habitat at high tide. Several unique features of this site may potentially influence the apparent lack of habitat use at low tide. This site is a fringing marsh, contiguous with the main channel of the Chickahominy River, and therefore does not exhibit the pronounced creekbank ecotone of marshes associated with smaller tidal creeks. The slope of the marsh is gradual throughout the lower intertidal and into the subtidal. This site is regularly flooded and the lower intertidal zone may remain submerged for 3 - 5 days during spring tides. During May -September, submerged vegetation encroaches up into the lower intertidal, where it achieves dense growth in shallow rivulets and intertidal pools. This shallow transition zone may support dense aggregations of marsh-dependent nekton, and is accessible to small fishes and macrocrustacans during most low tide events. Clearly, nekton such as grass shrimp and mud crabs preferentially select SAV habitat over marsh intertidal when available (Rozas and Odum, 1987b; 1987c); reduced stress, effective predation refuge, and profitable foraging among SAV would seemingly attract other nekton species as well. The intertidal pools and rivulets are larger, deeper and more abundant at Eagle Bottom; this is a function of the dominant emergent macrophyte present, Peltandra virginica. Nekton are not confined to individual microhabitats at Eagle Bottom, as the pools in the mid-to lower intertidal are often connected by small rivulets. Thus, nekton are able to move about the marsh surface, even when drained at low tide. This would certainly reduce the likelihood of stranding in individual pools; consequently the ability of pit traps to capture large numbers of nekton might be similarly reduced. In contrast, at Beaver Dam Creek, individual microhabitats are fewer in number, and tend to be isolated from one another. This feature increases the likelihood of concentrating nekton seeking intertidal refuge as the marsh drains. In salt marshes, intertidal refuges are generally less abundant and shallower, further restricting the size and abundance of organisms present. Despite this, abundance of nekton on the salt marsh surface, particularly grass shrimp, was high.
Rozas and Reed (1993) suggested that utilization of Gulf Coast marsh habitats may be enhanced relative to Atlantic coast wetlands as a result of longer average hydroperiod duration. Average monthly flooding duration of our salt marsh sites ranged from 13 - 28%; these values are considerably lower than those reported by Rozas for Gulf Coast marshes (39 - 68%). In general, flooding duration of the Chickahominy River marshes is comparable to that reported for Gulf coast marshes. However, the confounding factors discussed previously (microtopogaphy, effects of SAV habitat) may reduce the habitat value of the freshwater intertidal marsh surface (when vegetated subtidal habitats are available) in comparison to Gulf coast marshes.
Marked increases in abundance of mosquitofish at tidal freshwater sites and spotfin killifish at salt marsh sites were observed during 1993 relative to 1992. Each species was the second most abundant fish collected at each site during both years. Reasons for the coincident increase in abundance of these two species during the second year of the study are unknown. Design, location, number, and orientation of traps remained the same during both years. Mosquitofish were never collected from intertidal salt marsh sites during this study; however, this species does occur at the Virginia Coast Reserve in semi-permanent high marsh pools or ditches containing fresh of brackish standing water (pers. obs). These isolated habitats are rarely inundated by floodwaters. Spotfin killifish were common at upper intertidal locations at Hammocks Marsh and were abundant throughout the intertidal at Phillips Creek Marsh. In July, 1993 spotfin killifish outnumbered mummichogs in salt marsh collections. Most spotfin killifish collected (87 %) were adults. Kneib (1984) found all age classes of spotfin killifish present in the upper intertidal marsh at Sapelo Island, Georgia. Abundance of mummichogs, the most abundant fish species, was markedly greater at salt marsh sites during 1993, particularly at Phillips Creek, where no fishes were collected during April-May 1992. This suggests that spawning and recruitment success was enhanced early in 1993 relative to the same time period in 1992. Fishes and decapods were abundant early in the season at Hammocks Marsh during both years. Spawning by F. heteroclitus begins as early as mid-April at the Virginia Coast Reserve (this volume, Chapter III). Failure of one or more early cohorts may have led to the absence of nekton in April - May 1992 collections at Phillips Creek. Naked gobies were collected at all four marsh sites, but were most abundant at the lower intertidal of Hammocks Marsh. This species is known for its close association with oyster bars, and although gobies may forage extensively within the lower intertidal marsh at high tide, the presence of several large oyster bars in the subtidal adjacent to Hammocks Marsh most likely explains its prevalance at this site.
A single specimen of Fundulus diaphanus was collected at tidal freshwater marshes; this species is numerically dominant in collections from submerged vegetation beds, along with F. heteroclitus (Rozas and Odum, 1987a), and has been reported as abundant on the flooded marsh surface (McIvor and Odum, 1989). Unlike F. heteroclitus, however this species is apparently unable to successfully reside on the marsh surface when drained and retreats to the sub-tidal at low water. A single juvenile brown bullhead Ameiurus nebulosus was collected from a creekbank at Beaver Dam Creek in July 12; none were taken in traps in 1993, however, juvenile brown bullheads have occasionally been observed in natural intertidal pools at Eagle Bottom Marsh. Grass shrimp were markedly more abundant at tidal freshwater sites in 1993. Mud crabs were not taken from pit traps in 1992; however, they were present late in the season, along with grass shrimp, in 1993.
One obvious physico-chemical difference between years was that of salinity. Because 1992 was a relatively wet year, freshwater conditions were observed at all sampling dates. In contrast, 1993 was a drought year, and subsequently, the salt front penetrated far upriver. The presence of some saline water may have affected recruitment of decapods such as grass shrimp or mud crabs during 1993. Alon and Stancyk (1982) documented variations in clutch size, life span, growth and sex ratio of grass shrimp from estuarine habitats with different salinity regimes. They concluded that reproductive flexibility is the mechanism by which this species is able to exploit a wide range of estuarine environments. Although significant differences in blue crab abundance were not observed in pit traps, casual observations and anecdotal reports by local residents and sportfisherman suggest that abundance of adult blue crabs was unusually high in the tidal freshwater Chickahominy River in 1993. Juvenile blue crabs were consistently present, though never abundant, in salt marsh traps during both years of this study. Fitz and Weigert (1991) documented moderate utilization of intertidal marsh habitats at Sapelo Island, Georgia by both juvenile and adult blue crabs, particularly during summer.
The observed difference in age class composition of fishes between tidal freshwater and salt marshes may be explained by the presence or absence of SAV in the subtidal environment. In salt marshes, where SAV is absent in tidal creeks, marsh-dependent nekton use the marsh surface for a longer part of their life history as a predation refuge. In tidal freshwater marshes, sub-adult fishes may move into structurally complex submerged vegetation in the adjacent sub-tidal zone which provides abundant forage and effective predation refuge regardless of tide stage. This would appear to explain the predominance of early juveniles in fish collections from tidal freshwater. Late juvenile stages and adults of forage species such as Fundulus spp. are primarily concentrated in submerged vegetation habitats. Juveniles of many species which do not utilize the intertidal surface at low tide may be abundant in grass beds. In preliminary collections, during November 1991, two bluegill sunfish Lepomis macrochirus YOY were collected at Eagle Bottom Marsh. This suggests that additional species may utilize the intertidal marsh late in the season, although none were taken during 1992-1993. Grass shrimp, and to a lesser extent, mud crabs apparently shift habitat preference in autumn, as SAV beds die off. However, most species leave the area, either having achieved sufficient size to sucessfully move into deeper waters or seek out alternative permanant refuge, such as coarse woody debris habitats (Everett and Ruiz, 1993).
Species composition of the resident marsh-surface sub-community is similar in tidal freshwater and salt marshes, despite physico-chemical differences and variation in general community composition. Young-of-the-year of several ubiquitous marsh species, including Fundulus heteroclitus, Gobiosoma bosci, Palaemonetes pugio, and Callinectes sapidus numerically dominated collections from both study areas. These species are characterized by broad environmental tolerances, enabling them to reside on the surface of tidal marshes for all or part of their life history.
Flooding depth and duration were positively associated with enhanced nekton use in salt marshes; this relationship appears to be reversed in tidal freshwater marshes, possibly as a result of the seasonal presence of submerged aquatic vegetation contiguous with low intertidal zone habitats and/or site-specific microtopographic features. Seasonal presence or absence of SAV may also influence patterns of marsh surface utilization by species characterized by strong temporal patterns of abundance in tidal freshwater, such as grass shrimp. Availability of SAV in subtidal habitats may also determine size/age class structure in marsh surface finfish communities; markedly greater abundance of adult fishes are present on the surface of salt marshes, which lack SAV in the adjacent subtidal zone.
Fish assemblages of mid-Atlantic tidal freshwater wetlands are dominated by warm freshwater species co-occuring with a smaller number of estuarine and marine transients which may use tidal freshwater habitats as nurseries or feeding grounds. Within a tidal freshwater marsh, individual species may be separated due to habitat requirements such that no one collection gear will be adequate to effectively sample the entire community. Sampling fishes in tidal freshwater marshes is constrained by environmental features which often render conventional collection gear ineffective. Dense emergent and submerged vegetation, soft mud substrates, and often unpredictable tides are features of the tidal freshwater marsh which contribute to sampling difficulty. Alternative sampling gear has been developed in order to address this problem, and recent studies have documented utilization of tidal freshwater wetlands by a variety of warmwater resident and estuarine migrant fish species (Serafy et al., 1988; McIvor and Odum, 1986; 1988; Rozas and Odum, 1987a; 1987b; Rozas, McIvor and Odum, 1988). I present here a list of 38 species compiled from fish habitat utilization studies conducted in tidal freshwater marshes contiguous with the Chickahominy River, Charles City County, Virginia from 1983 - 1993 (Table 5.1). Fishes have been sampled from several marsh sub-environments including vegetated creek bottoms, unvegetated creek bottoms, shallow vegetated mudflats, erosional and depositional creekbanks, and the lower and upper intertidal marsh surface. Sampling techniques used in these studies include the flume net, developed specifically for use in the Chickahominy River marshes (McIvor and Odum, 1986), pop-nets, dip-netting in submersed plant beds, throw traps, a drop sampler, intertidal pit traps, gill nets, electrofishing, angling, a pull-up channel net, and seining.
Flume nets:
Flume nets were used from 1983 - 1986 to quantitatively estimate abundance of resident fishes and macrocrustaceans using the flooded marsh surface (McIvor and Odum, 1988; Rozas and Odum, 1987b; Rozas, McIvor and Odum, 1988). Most species collected (87%) were taken using this gear type. The flume net captures fishes leaving the flooded marsh surface on ebb tides in a removable cod end. Flume nets are clearly effective for sampling a broad spectrum of the fish community which may use the marsh edge/flooded marsh surface. However, several species reported here (primarily large piscivores) were not represented in flume net collections. The flume does not account for lateral movement of fishes across the marsh surface and it may integrate fish samples collected from the marsh surface with those occupying submersed vegetation at the marsh edge - subtidal ecotone; nonetheless, it is probably the most effective technique to date for sampling the marsh-dependent fish community.
Pop-Nets:
Pop-nets have been used previously to sample fishes in dense submersed vegetation (Serafy et al., 1988). Small pop-nets (2.75 m2) were used to collect fishes at a lower intertidal marsh site at the Chickahominy River in early Fall, 1990. Net frames were constructed of 1" diameter PVC pipe. The bottom frame was filled with sand and the buoyant upper frame was covered with foam pipe insulation. A hook and eye release mechanism was triggered manually by two workers standing on catwalks. Inland silversides, tesselated darters, and juvenile bluegill sunfish were the only three species to be taken in pop-nets. Daggerblade grass shrimp (Palaemonetes pugio) were abundant on the marsh surface in Fall and were consistently taken in pop-net samples Dense emergent vegetation and relatively shallow water depth (and unpredictable wind-driven tides) contributed to difficulties with this gear on the lower intertidal marsh surface. Pop-nets would probably be effective at collecting young-of-the-year in submersed plant beds at the Chickahominy River marshes. However, a serious drawback to pop-nets is the time spent installing and then later deploying the net. Throw/drop traps are probably a superior alternative to pop-nets and have been used by previous investigators in shallow vegetated freshwater and marine habitats (Kjelson and Johnson, 1973; Kushlan, 1981; Chick et al., 1992; Sogard and Able, 1991; Baltz et al., 1993)
Throw/drop traps and dip-netting:
A 1 m2 aluminum throw trap was used to sample fishes in submersed plant beds in 1985. This technique was effective at capturing small fishes resident in the beds, most of which (93%) were young-of-the-year (Rozas and Odum, 1987). A 30 cm diameter plexiglass cylinder was used as a drop sampler to collect epiphytic invertebrates in submersed plant beds in late Summer/early Fall 1993. Larvae and juveniles of several marsh-dependent fish species (white perch, bluegill sunfish, American eel, banded killifish, mummichogs) were taken incidentally in these collections. D-frame dip-nets (1 mm nytex mesh) have been used to obtain qualitative samples of YOY fishes in submersed plant beds. The dense vegetative structure of the beds apparently inhibits escape by resident fishes and dip-netting can be a effective means of collecting in this environment.
Pit traps:
Pit traps (Kneib, 1984; Talbot and Able, 1984) have been used to compare relative abundance and distribution patterns of marsh-resident species at tidal freshwater and salt marsh sites in Virginia since 1991. Traps consisted of an 11.4 liter plastic basin which was fitted into a shallow pit dug into the marsh surface. A nylon mesh liner (1.3 mm mesh) was fitted into the basin and held in place with PVC L-brackets which were inserted into the marsh surface around the perimeter of the basin. Generally, only species which spend all or most of their life history on the marsh surface (i.e. Cyprinodontidae, Gambusia, grass shrimp), are likely to be abundant in pit traps collections. However, naked gobies, juvenile blue crabs (Callinectes sapidus) and small crayfish (Cambarus robustus) were often collected on the intertidal freshwater marsh surface using pit traps.
Gill-nets/ electrofishing:
Mono-filament gill nets (2.54 and 5.08 cm mesh) were used to collect marsh-dependent fishes leaving tidal freshwater marsh creeks during 1992 - 1993 (see Appendix III). Although strongly size-selective, this technique was effective at collecting relatively large, adult piscivores which are not likely to utilize marsh edge or surface habitats. (i.e. largemouth bass, white perch, yellow perch, channel catfish). The smaller mesh nets were effective at capturing young-of-the-year of these and other species as they emigrated from tidal creeks in early Fall. On several occasions, angling was used to supplement gill net collections in creeks. Electrofishing with DC pulsed current was conducted in several tributaries of the Chickahominy River in Fall, 1985. Although more species were collected in gillnets, electrofishing was reasonably effective at sampling and identifying the piscivore guild (McIvor and Odum, 1988).
Seines/channel net:
Various seines with mesh sizes of 3 - 9 mm have been used, with some success in tidal freshwater. Quantitative seining is difficult, and suitable locations for seining are limited due to soft substrates and the prevalence of coarse woody debris along forested shorelines. Despite these difficulties, a number of species (26%) have been collected by seining, and the technique can be useful as a supplement to other collection techniques. As an alternative to seining, a 4 x 2 m pull-up channel net (5 mm ace nylon mesh) was constructed in 1990 in order to sample small tributary tidal freshwater marsh creeks. The pull-up net was deployed by two operators standing on opposite creekbanks and was effective at sampling small demersal fishes (i.e. tesselated darters) present in marsh creeks.
Drawing from experiences in the Chickahominy River marshes, the most effective combination of sampling techniques for use in tidal freshwater wetlands would probably be use of flume nets, perhaps in conjunction with pit traps in the upper intertidal and gill netting in adjacent deeper waters to collect species which do not utilize the flooded marsh surface. Throw/drop samplers are most effective for sampling small, primarily juvenile fishes utilizing submerged plant beds as habitat. Recently, two new quantitative sampling techniques have been developed for use in tidal marshes. The flume weir (Kneib, 1991) quantitatively samples nekton within a 100 m2 area of intertidal marsh surface but may be prohibitively expensive and labor-intensive for most applications. The bottomless lift-net (Rozas, 1992) combines features of the pop-net, the pull-up channel net, and pit traps and is an effective and economical means of quantitatively sampling flooded intertidal marsh habitats. This gear would be ideally suited for continued investigations at the Chickahominy River and other tidal freshwater wetlands of the U.S. east coast.
Meiofauna were sampled from intertidal pool and vegetative hummock microhabitats at a tidal freshwater marsh on the Chickahominy River, Virginia. Nematodes, ostracods, tardigrades, oligochaetes (Naididae), copepods (Harpacticoida and Cyclopoida), and the sabellid polychaete Manayunkia were numerically dominant in monthly collections. Total meiofaunal densities ranged from 169 individuals 10 cm-2 (low marsh pools, April) to 13832 individuals 10 cm-2 (low marsh hummocks, September). Highest densities of total meiofauna were observed in August-September, coincident with recruitment of oligochaetes and Manyunkia to low marsh hummocks. Nematodes were generally abundant in all seasons and represented 37% of total meiofauna collected. Nematode densities were significantly greater on hummocks (p = 0.0001). Ostracods were significantly more abundant in pools. Greatest densities of ostracods were observed in May. Tardigrades were abundant November through March, and September. Significantly greater abundance of tardigrades was observed on hummocks. Harpacticoid copepods were abundant from December through April and cyclopoids were abundant from May through September (low marsh only).
Intertidal freshwater meiofauna may represent an important, yet previously undescribed, trophic link in tidal freshwater wetlands; ostracods, copepods and other meiofauna are frequently consumed by young-of-the-year cyprinodonts (Fundulus spp.) and other sub-adult nekton utilizing the surface of tidal freshwater wetlands as a nursery area.
Benthic invertebrate communities of tidal freshwater wetlands have received little attention from estuarine ecologists. Along the mid-Atlantic coast, tidal freshwater wetlands represent an important transition zone between salt-brackish marshes and non-tidal freshwater environments. High primary productivity and pronounced seasonality of diverse plant communities are characteristic of tidal freshwater wetlands (Odum et al., 1984) Several economically important finfish species depend on tidal freshwater wetlands as nursery habitat; consequently these areas provide important forage for larval and juvenile life stages of estuarine dependent fishes.
Meiofauna are often the dominant prey item selected by larval and juvenile fishes in shallow estuarine habitats (Smith and Coull, 1987; Nelson and Coull 1989; Feller et al., 1990; Yozzo and Odum, 1993). Previous research in southeastern U.S. salt marshes has shown that intertidal meiofauna residing in the upper few millimeters of surface sediment, particularly harpacticoid copepods and their nauplii, are the primary prey item for young-of the-year spot Leiostomus xanthurus (Ellis and Coull, 1989). In these salt marsh ecosystems, harpacticoids may comprise up to 17% of total meiofauna. However, in gut contents, they may comprise up to 70% of total prey consumed by juvenile spot (Nelson and Coull, 1989). Yozzo and Odum (1993) reported that epiphytic and benthic meiofauna (ostracods, cyclopoid copepods, dipteran larvae) were significant components of the diets of post-larval and juvenile banded killifish Fundulus diaphanus in a Hudson River tidal freshwater wetland. Despite the potential trophic significance of meiofauna in estuarine wetland habitats, little information is available on community composition, seasonal abundance and distribution of meiofauna in tidal freshwater wetlands.
Surface topography of tidal freshwater wetlands may be strongly determined by the growth form of intertidal macrophytes. Distinct vegetative hummocks are present in the lower intertidal zone of tidal freshwater marshes characterized by arrow-arum Peltandra virginica. Shallow intertidal pools and rivulets form between P. virginica hummocks and retain standing water at low tide. I hypothesized that species composition and densities of resident meiofauna would differ between pool and hummock microhabitats as a result of variation in inundation time. Specifically, I hypothesized that: 1) hummocks would support greater densities of dessication-resistant meiofauna. 2) pools would support taxa characteristic of sub-tidal habitats, and 3) pools would be characterized by a greater number of species. Changes in meiofaunal abundance and species composition within and between microhabitats and intertidal zones of a tidal freshwater marsh were monitored monthly.
This study was conducted at Eagle Bottom Marsh, a fringing tidal freshwater marsh contiguous with the Chickahominy River, a tributary of the James River sub-estuary of the Chesapeake Bay, in Virginia (Fig. 6.1). Previous research at this site has focused on subsurface hydrology and porewater nutrient dynamics (Harvey and Odum, 1991, Chambers and Odum, 1991). Eagle Bottom Marsh is regularly flooded with a gradually sloping topographic profile. This site has been instrumented with a Qualimetrics Richards-Type water level recorder since May 1993. Duration of average monthly flooding is > 70%. Average depth of flooding is > 50 cm. Mean tidal amplitude at this location is 0.7 m and mean salinity is generally < 0.5 ppt, except during regional drought conditions (McIvor and Odum, 1988). The lower intertidal zone is vegetated entirely by arrow-arum Peltandra virginica. The upper intertidal supports a mixed community of P. virginica, northern wild rice Zizania aquatica, giant cutgrass Zizaniopsis miliacea and marsh hibiscus Hibiscus moscheutos. Submersed vegetation (Ceratophyllum demersum) is abundant from May through October in the extreme lower intertidal to subtidal zones.
Preliminary sampling was conducted in November 1991 in order to determine meiofaunal community composition and microhabitat distribution. A 100 m2 plot was established in the lower intertidal zone. We collected 30 cores (1.5 cm diameter, 4 cm depth) from pools and 30 cores from hummocks adjacent to randomly selected points within the plot. Cores were extruded into 100 ml glass vials and preserved in a 10% buffered formalin/rose bengal solution. In the laboratory, samples were seived through a 63 u m mesh, identified, and enumerated. Microcrustaceans were identified to genera or species. Annelids and insects were identified to family. All other organisms were identified to phyla. I tested for differences in abundance of total meiofauna and 7 numerically dominant taxa (Nematoda, Ostracoda, Tardigrada, Oligochaeta, Manyunkia, Harpacticoida, Cyclopoida) between pool and hummock microhabitats using analysis of variance (ANOVA). All abundance data were log-transformed prior to analysis (Sokal and Rohlf, 1981). Statistical analyses were performed using SuperANOVA software for the Macintosh PC (Abacus Concepts, 1989).
Monthly sampling was initiated in November 1992 and continued through October 1993. In order to compare seasonal abundance, composition and distribution along an elevation gradient, meiofauna were sampled from high and low marsh sampling plots at low tide. Six replicate cores were collected from pool and hummock microhabitats within each zone. A total of 24 cores were collected on each sampling date. Surface water salinity and temperature were recorded on each sampling date.
I tested for differences in log-transformed abundance of total meiofauna and 7 dominant taxa using a repeated-measures analysis of variance with ZONE (high marsh vs. low marsh) and MICROHABITAT (pools vs. hummocks) as main effects and SAMPLING DATE as the repeated factor.
Six invertebrate phyla were represented among meiofauna collected at Eagle Bottom Marsh (Table 6.1). The dominant meiofaunal taxa present were nematodes, ostracods (at least 5 genera), tardigrades, oligochaetes (Naididae), the sabellid polychaete Manyunkia spp. and copepods (harpacticoid and cyclopoid). Nematodes and ostracods together comprised 58% of total meiofauna collected. Additional taxa present included rotifers, turbellarians, water mites (Hydracarina), chironomid larvae (temporary meiofauna), and cladocerans (Bosmina longirostris). Bryozoan statoblasts were often observed in samples taken from intertidal pools.
Surface water salinity ranged from 0 ppt (November 1992 - July 1993) to 3 ppt (September 1993). Noticeable penetration of the salt front into the upper estuary occurred in 1993, a relatively dry year. Temperature ranged from 8[[ring]] C (December 1992) to 31[[ring]] C (July 1993).
Analysis of the 60 cores taken in November 1991 revealed no significant differences in total meiofaunal density between pools and hummocks. Similarly, taxonomic composition in pools and on hummocks was virtually identical, with 10 taxa present both in pools and on hummocks. Although no difference in total density was observed, abundances of individual species varied significantly between the two microhabitats (Fig. 6.2). Nematodes were significantly more numerous in hummocks (p = 0.0161), while ostracods, Manyunkia spp. and harpacticoid and cyclopoid copepods were significantly more numerous in intertidal pools (p < 0.01). Abundance of tardigrades was not significantly different between microhabitats. Early instar chironomid larvae were present, although not particularly abundant, in both pool and hummock samples.
Five additional taxa were recovered from seasonal collections during 1992 - 1993, although none were numerically important. Turbellarians, rotifers, and water mites were occasionally collected from both pools and hummocks. Bosmina longirostris was present in pools from May through August and in November. This species was also collected from hummocks in August. The cytherid ostracod Ilyocypris gibba was collected from a high marsh pool in May. Total meiofaunal density ranged from 169 10 cm-2 (low marsh pools, April) to 13832 10 cm-2 (low marsh hummocks, September). In the high marsh, total meiofauna densities were generally constant throughout the year, with slightly greater numbers in May, resulting from a recruitment pulse of juvenile ostracods in high marsh pools (Fig. 6.3). In the low marsh, a significant total abundance peak observed in August - September resulted from recruitment of Manyunkia spp., and naidid oligochaetes, respectively, on hummock surfaces. Mean naidid densities on hummocks exceeded 3500 individuals 10 cm-2, and Manyunkia densities exceeded 9000 individuals 10 cm-2 at this time (Table 6.2). This recruitment pattern resulted in significant differences between microhabitats for total abundance, overall (p = 0.0001). Total meiofauna densities did not significantly differ between high and low marsh zones.
Nematodes were most abundant from November through May and were slightly more abundant in the high marsh (Fig. 6.4). Significantly greater nematode abundance was observed throughout the year on hummocks at both high and low marsh zones (p = 0.0001). Densities ranged from 9 10 cm-2 (low marsh pools, July) to 2815 10 cm-2 (high marsh hummocks, November). Mean annual density of nematodes for all habitats combined was 803 10 cm-2. This estimate is comparable to Coull's (1985) estimate of 856 10 cm-2 from a North Inlet, South Carolina mud flat. Densities decreased rapidly from May to October. Low marsh nematode density fluctuated widely, with peak numbers observed in April and September.
Ostracod density was highest in marsh pools, especially in May, when numerous early instars were present in samples. Ostracod densities ranged from 0 to 3469 10 cm-2 with a mean annual abundance of 461 10 cm-2 across all habitats. Overall, significantly greater ostracod abundance (p = 0.0001) was observed in the low marsh (Fig. 6.5). Cyclocyprids, primarily Physocypria spp. dominated the ostracod fauna of Eagle Bottom Marsh. Candona spp., Cypridopsis vidua, and Darwinula stevensoni were also abundant.
Tardigrades occurred consistently in samples during most months, with the exception of July. Greatest density of tardigrades (6008 10 cm-2) was observed on hummocks in September. Mean density of tardigrades was 803 10 cm-2. Copepods (both harpacticoid and cyclopoid) were consistently present in samples, although they rarely reached densities greater than 500 10 cm-2. Mean annual densities were 44 10 cm-2 and 39 10 cm-2 for harpacticoids and cyclopoids, respectively. Individually, or combined, copepod densities at Eagle Bottom Marsh were markedly lower than that reported from salt marsh sites in South Carolina (Coull, 1985) and Louisiana (Fleeger, 1985). Harpacticoids, represented by Canthocamptus spp. and Bryocamptus spp. were most abundant from November through April, while cyclopoids (Eucyclops agilis) were most abundant from April through September. Female harpacticoids were gravid in January and February; female cyclopoids bore eggs throughout the year.
Virtually all previous research on the dynamics of marsh meiofauna have been conducted in salt marshes, primarily on the southeast and gulf coasts of the U.S. Long-term studies of meiofaunal population dynamics at North Inlet, South Carolina revealed little year-to-year variability. However, distinct seasonality was evident, particularly at muddy-bottom sites, where active predation by juvenile finfish may strongly depress abundance of prey species (Eskin and Coull, 1987). At the Chickahominy River site, distinct differences in species-specific densities were evident between high and low marsh habitats. Nematodes experienced a steady decline from spring to early summer in high marsh pools and hummocks, yet in the low marsh, nematode abundance was highly variable throughout the year. Fitzhugh and Fleeger (1985) observed significant numbers of nematodes, along with harpacticoids and meiofaunal polychaetes, in the diets of gobiid fishes in a Mississippi delta marsh. Nematodes have occasionally been observed in guts of F. heteroclitus at Chickahominy River marshes, however, they do not appear to be an important component of the diet of these marsh resident fishes. One gobiid species, Gobiosoma bosci, is seasonally abundant in Chickahominy River marshes. If predation by young-of-year fishes is significant in controlling nematode abundance this would explain the decline in the high marsh, where fishes may feed around hummocks and in intertidal pools. Apparently, different processes control nematode abundance in high marsh and low marsh habitats at Eagle Bottom Marsh.
Palmer (1980) documented highest abundance of the harpacticoid Microarthridion littorale in the intertidal zone at North Inlet in July, while density of a subtidal population peaked in October. She suggested that flooding duration, as determined by topographic profile of the marsh, in addition to variability in food resources and competitive pressure, may have been responsible for the observed variation in life history and abundance of this species. At Eagle Bottom Marsh, harpacticoid abundance decreased from May to October in both high and low marsh habitats. Cyclopoid densites were much more variable; however, a spring abundance peak was followed by relatively low densities in June-July, coincident with the greatest abundance of larval and juvenile fishes on the marsh surface. Harpacticoid and cyclopoid copepods are consistently present in guts of larval and juvenile cyprinodonts (Fundulus spp.) and other young-of-year finfish utilizing the surfaces of Chickahominy River marshes as a forage site (pers. obs.).
Seasonality of meiofaunal annelids has been reported from South Carolina salt marshes (Bell, 1979, Bell, 1982). Juveniles of the sabellid Manyunkia aesturina, and the spionid Streblospio benedicti comprised a significant percentage of total meiofauna at North Inlet in autumn. Similar abundance peaks of Manyunkia spp. and naidid oligochaetes were observed at Eagle Bottom Marsh in August and September 1993.
In southeastern U.S. salt marshes, nematodes are the primary constituent of the meiofauna, representing over 70% of the community (Bell, 1979). Although nematodes are the numerically dominant taxa at Eagle Bottom Marsh, they represented only 37% of total meiofauna collected in our study. Munson (1985) reported that nematodes represented up to 71% of total meiofauna at Ducking Stool Point, a James River, Virginia tidal freshwater marsh. However, her study was restricted to three sampling dates in April, July, and September and may not reflect the marked temporal variability in abundance as indicated at Eagle Bottom Marsh. In addition, her study did not include annelids; therefore, nematode abundance relative to other taxa may have been overestimated. Ostracods represented 21% of total meiofauna at Eagle Bottom Marsh; this is comparable to Munson's (1985) study where ostracods comprised up to 20% of total meiofauna, excluding annelids. In tidal freshwater wetlands, ostracods appear to represent an important prey item for sub-adult fishes. Ostracods are abundant in stomachs of juvenile mummichogs Fundulus heteroclitus and bluegill sunfish Lepomis macrochirus from Chickahominy River wetlands. Yozzo and Odum (1993) documented the importance of ostracods and other meiofaunal taxa (including the cyclopoid Eucyclops agilis) as prey for juvenile and adult banded killifish Fundulus diaphanus in a Hudson River tidal freshwater wetland. Annelids (Manyunkia spp. and Naididae) represented 27% of total meiofauna at Eagle Bottom Marsh. Diaz et al., (1978) reported Manyunkia spp. as rare at Windmill Point, a James River tidal freshwater marsh, although other annelids (Oligochaeta) comprised 10% of total meiofauna. Copepods (harpacticoid and cyclopoid) represented 4% of total meiofauna at Eagle Bottom Marsh. However, Munson (1985) reported that copepods represented up to 25% of total meiofauna from her James River marsh. This discrepancy may be explained by her inclusion of subtidal creek sampling stations, where copepods may have been more abundant, in addition to intertidal marsh sites. Diaz et al., (1978) reported that copepods comprised 9% of total meiofauna at Windmill Point.
Previous workers have postulated that densities of marsh meiofauna may vary significantly as a result of tidal stage. I collected samples only at low tide, when hummocks were exposed. Most pools retained standing water of a few cm depth. Palmer and Brandt (1981) found significantly greater densities of copepods at slack high and low tide than at flooding or ebbing tides from intertidal and subtidal sampling sites at North Inlet. Coull and Feller (1988) determined that there was generally no detectable difference in high or low tide abundance of copepods along an intertidal marsh transect at North Inlet and recommended that meiofaunologists continue to sample intertidal habitats at low tide in order to estimate maximum abundance of a species. Surficial sediments at Eagle Bottom Marsh are highly organic and loosely consolidated. Considerable sediment (and presumably meiofaunal) resuspension occurs during twice-daily flood tides. In consideration of the dynamic nature of the substrate and the unique qualities of the intertidal freshwater environment, it would probably be worthwhile to investigate the role of tide stage in determining the abundance and distribution of freshwater intertidal marsh meiofauna.
Micro and mesoscale distribution patterns of salt marsh meiofauna are well-documented. Fleeger et al., (1990) determined that controlling processes on the scale of several cm2 dictated aggregation dynamics of meiofauna from a Louisiana intertidal mudflat. Phillip and Fleeger (1985) found significant meso-scale variation in meiofaunal abundance between habitats, within locales among habitats, and among months sampled. Similarly, I found significant differences in abundance for total meiofauna and for individual taxa in my comparison of marsh levels and microhabitat comparison. I also observed significant temporal main effects for all numerically important taxa.
Previous workers have documented enhanced abundance of intertidal salt marsh meiofauna associated with individual culms of Spartina alterniflora. Rader (1984) reported nematode abundances three times greater in sediment cores containing individual Spartina alterniflora culms compared to unvegetated areas in a North Carolina salt marsh. Osenga and Coull (1983) also reported positive correlation between nematode abundance and Spartina culms, suggesting that nematodes are concentrated around micro-oxygenated zones produced by live Spartina roots. Peltandra virginica hummocks at Eagle Bottom Marsh are primarily composed of dense, fibrous root material, under a few cm of highly organic substrate. If nematodes are, in fact, attracted to micro-oxygenated zones surrounding live root material, this would possibility explain the consistently greater densities of nematodes observed on hummocks. Tardigrades were significantly more abundant on hummocks from November 1992 through November 1993. Naidids were significantly more numerous on hummocks, primarily as a result of the September abundance peak. Although Eagle Bottom Marsh is inundated > 70% of the time, twice daily exposure of hummock surfaces results at low tide. Nematode eggs are highly resistant to dessication via anhydrobiosis (Pennak, 1989). Tardigrades are well-represented in ephemeral or semi-aquatic habitats. As a group, they are well-adapted to dessication stress and will readily undergo repeated episodes of anhydrobiosis depending upon environmental conditions (Pennak, 1989). The Naididae are considered to be strictly aquatic (Pennak, 1989).
Manyunkia spp. were significantly more abundant in pools in November 1991. However, despite the extremely high densities of Manyunkia recruiting onto hummocks in August, seasonal sampling revealed no significant habitat preference for this species. Ostracods were significantly more numerous in intertidal pools at all times and were the only group to demonstrate an obvious preference for the pool microhabitat during seasonal sampling. Copepods (harpacticoid and cyclopoid) were significantly more numerous in pools in November 1991. No significant habitat preference was demonstrated for copepods during seasonal sampling. Despite the observed microhabitat preferences, species composition of pools and hummocks was virtually identical throughout the year.
In salt marshes, the spatial distribution and areal density of meiofauna may be influenced in part by disturbance/predation by epifaunal and/or benthic macroinvertebrates. Coull et al. (1979) suggested that observed zonation patterns of harpacticoid copepods at North Inlet, South Carolina may be due in part to activity of macrofaunal disturbers/predators. Bell and Coull (1978) found that densities of harpacticoids, oligochaetes, and polychaetes were positively correlated with exclusion of the grass shrimp Palaemonetes pugio. Grass shrimp are seasonally abundant on the surface of Virginia tidal freshwater marshes in late summer and autumn (Rozas and Odum, 1987). As submersed vegetation beds in the adjacent creeks die-off, grass shrimp emigrate from the sub-tidal to the intertidal, seeking refuge on the vegetated marsh surface. At this time, grass shrimp may potentially influence the abundance of certain meiofaunal taxa. Data presented here do not suggest such an occurrence at Eagle Bottom Marsh, however, as total meiofaunal density is highest at this time, resulting from abundance pulses of meiofaunal annelids (Naididae and Manyunkia spp.)
The intertidal macroinvertebrate fauna at Eagle Bottom Marsh is dominated by the amphipod Gammarus fasciatus. Isopods Caecidotea spp. and Cyathura polita are commonly observed swimming in pools and, along with G. fasciatus are often collected in larval and juvenile fish traps. Juvenile blue crabs Callinectes sapidus and mud crabs Rhithropanopeus harissi are frequently encountered in intertidal pools. Additional taxa present include the amphipod Corophium spp., oligochaetes (Tubificidae, Enchytraidae), leeches (Placobdella ornata, Mooreobdella spp.), Dipteran larvae (Chironomidae, Ceratapogonidae), Gastropods (Physa integra, Gyraulus parvus), Bivalves (Pisidium spp.), Megalopterans (Sialis spp.) and Odonates (Somatochlora spp., Enellagma spp.). Several of these taxa are potential predators on meiofauna in tidal freshwater habitats. Sialids are actively predacious and are known to ingest annelids and microcrustaceans (Peckarsky et al., 1990). Numerous Dipteran species are known to actively prey upon microcrustaceans (Goulden, 1971; Peckarsky et al., 1990). Odonates are active predators, and feed extensively upon small crustaceans and oligochaetes (Peckarsky et al., 1990).
Biogenic structures associated with macroinfaunal burrowing activity may function as aggregation sites for salt marsh meiofauna. Bell et al., (1978) documented enhanced nematode density associated with fiddler crab Uca pugnax burrows in a South Carolina salt marsh. Strong effects of fiddler crab deposit feeding were demonstrated experimentally for nematodes and meiofaunal crustaceans in a New england salt marsh (Hoffman et al., 1984). One species of fiddler crab (Uca minax) occurs in Virginia tidal freshwater marshes. However, it is apparently secretive or uncommon, as it is observed only infrequently at the Chickahominy River study sites. Evidence of burrowing activity by this species is rare and is generally restricted to depositional creekbanks. Thus, crab burrows are unlikely to represent a significant determinant of meiofaunal distribution/abundance in tidal freshwater wetlands.
Clearly, much remains to be learned about the dynamics of meiofaunal populations in intertidal freshwater habitats. The importance of hydrodynamics, tide stage, and sediment resuspension in determining the distribution of intertidal freshwater meiofauna merits investigation. The role of macroinvertebrate and finfish predation on the abundance and distribution of intertidal freshwater meiofauna warrants intensive study. Experimental manipulations, along with dietary studies of suspected macroinvertebrate and finfish predators, may provide insight on the influence of macrofaunal predators in controlling the spatial distribution and density of meiofaunal prey on the surfaces of tidal freshwater wetlands.
In the preceding chapters, I have examined the influence of environmental parameters (primarily tidal flooding regime and surface topographic features) on the dynamics and spatial/temporal abundance patterns of marsh resident nekton communities. In general, the habitat value of tidal freshwater wetlands has been inadequately documented in comparison to that of coastal salt marshes. Previous studies have generally been confined to a specific ecosystem type and inter-site comparison is constrained by a lack of methodological standardization.
In my study, comparisons of nekton abundance/distribution were made across individual topographic strata and between marshes with varying flooding regimes at tidal freshwater and salt marsh study sites in Virginia. Standardized cross-site comparisons of nekton habitat use in estuarine intertidal environments are unprecedented in the existing literature. In addition, the dynamics and temporal/spatial distribution of a marsh meiofauna community were examined at a tidal freshwater marsh, in order to determine community composition and patterns of meiofaunal distribution with respect to season and microhabitat. Detailed investigations of intertidal freshwater meiobenthos are lacking, particularly with respect to microtopographic variation and its effect on the distribution and abundance of marsh meiofauna. Certain meiofaunal taxa are an important food source for sub-adult nekton in intertidal freshwater habitats and quantifying the abundance and relative availability of these prey taxa is an important first step towards assessing the transfer of marsh secondary production through intermediate and higher level consumers.
The following main conclusions have been derived from this study:
Young-of-the-year of several ubiquitous marsh species, including Fundulus heteroclitus, Gobiosoma bosci, Palaemonetes pugio, and Callinectes sapidus numerically dominated intertidal collections from both study areas. These species are characterized by broad environmental tolerances, enabling them to reside on the surface of tidal marshes for all or part of their life history. These findings have implications for management of natural or altered intertidal marsh habitats and may be of importance in the development of design criteria for mitigated marshes, particularly if management goals are oriented towards optimization of habitat use by a particular "target species" of ecological or economic significance (i.e. blue crab).
Abundance of mummichog Fundulus heteroclitus YOY was greater at a regularly flooded mainland salt marsh site (Hammocks Marsh) in comparison to an irregularly flooded high marsh (Phillips Creek Marsh). This is in agreement with the concept that frequent flooding enhances growth and survival of marsh-resident nekton. In general, I observed greater relative abundance of mummichog YOY at creekbank and low marsh stations relative to the upper intertidal at both regularly and irregularly flooded marshes.
In addition, marsh-resident finfish (Fundulus spp.) were more abundant at a mainland salt marsh relative to a back-barrier marsh at the Virginia Coast Reserve. Between-site differences in elevation and hydroperiod and the relative availability of high marsh habitat are factors which may potentially influence the observed patterns of abundance.
In tidal freshwater, the intertidal marsh surface adjacent to submerged vegetation (Eagle Bottom Marsh) was virtually unused by resident nekton at low tide throughout most of the sampling season. However; a tidal freshwater marsh characterized by a relatively short hydroperiod (Beaver Dam Creek) was utilized extensively by resident sub-adult nekton at low tide. It is postulated that the presence of submerged vegetation contiguous with the lower intertidal zone at Eagle Bottom Marsh affected habitat use by providing an alternative high-quality refuge habitat in the lower intertidal-subtidal transition area at this site.
Seasonal presence or absence of SAV may also influence utilization of the intertidal marsh surface by species characterized by strong temporal patterns of abundance in tidal freshwater, such as grass shrimp. As SAV beds rapidly senesce during autumn, grass shrimp emigrate to the intertidal marsh surface in large numbers, seeking an alternative refuge/foraging habitat. Availability of SAV in subtidal habitats may also determine size/age class structure in marsh surface finfish communities; markedly greater abundance of adult fishes are present on the surface of salt marshes, which lack SAV in the adjacent subtidal area.
Drawing from experiences in the Chickahominy River marshes, the most effective combination of sampling techniques for use in tidal freshwater wetlands would probably be use of flume nets, perhaps in conjunction with pit traps in the upper intertidal and gill netting in adjacent deeper waters to collect species which do not utilize the flooded marsh surface.
At the Virginia Coast Reserve, spotfin killifish Fundulus luciae were frequently collected in intertidal pit traps and are apparently not uncommon in high marsh habitats at the VCR. As suggested by previous investigators, the purported rarity of F. luciae in mid-Atlantic salt marshes is probably due to under-representation by conventional sampling techniques combined with specific habitat requirements.
Nematodes, ostracods, tardigrades, oligochaetes (Naididae), copepods (Harpacticoida and Cyclopoida), and the sabellid polychaete Manayunkia were numerically dominant in monthly meiofauna collections from Eagle Bottom Marsh, on the Chickahominy River. Highest densities of total meiofauna were observed in August-September, coincident with recruitment of oligochaetes and Manyunkia in the low marsh. Nematodes were abundant in all seasons and represented 37% of total meiofauna. Ostracods and copepods were significantly more abundant in intertidal pools; nematodes, naidids, and Manyunkia spp. were significantly more abundant on vegetative hummock surfaces.
Ostracods, copepods and other meiofauna are frequently consumed by young-of-the-year Cyprinodonts (Fundulus spp.) and other sub-adult nekton utilizing the surface of tidal freshwater wetlands as a nursery area. The availability of meiofaunal prey within microhabitats which are utilized extensively by sub-adult nekton (i.e. intertidal pools) may strongly determine foraging patterns and recruitment success of nekton species which rely on freshwater intertidal habitats.
Future research on tidal marsh trophodynamics should focus on predator-prey interactions and the transfer of secondary production from intertidal meiofauna (and small macrofauna) to nektonic consumers. Intertidal predator-prey dynamics have been well-documented in salt marshes; however, little information is currently available on predator-prey dynamics in low-salinity intertidal habitats.
A first step towards quantifying trophic linkages and energy transfer dynamics in estuarine wetlands would require reliable estimates of secondary productivity by prey taxa (infaunal and epifaunal invertebrates) occupying intertidal habitats. This information is currently unavailable for low-salinity estuarine habitats and has received only moderate attention in salt marshes. Productivity estimates should then be coupled with quantitative estimates of nektonic consumer densities and rates of consumer movements within and between shallow estuarine sub-environments (i.e upper intertidal - lower intertidal - shallow subtidal) in order to ultimately quantify export of detrital energy from intertidal wetlands to nearshore coastal environments. A standardized, comparative approach encompassing shallow habitats located throughout the estuarine salinity gradient would ensure applicability and reliability of the resulting data. The information presented in this dissertation represents the foundation for continued research on habitat use and trophic dynamics of intertidal wetland habitats; however, additional work is needed in order to clearly define the linkages between secondary productivity, habitat utilization, and energy transfer. Future research emphasis in this direction would result in information that would be of direct benefit to environmental scientists and coastal resource managers involved in the regulation of estuarine living resources and habitats.
Abacus Concepts. 1989. SuperANOVA. Abacus Concepts, Inc. Berkeley, CA.
Able, K.W. 1984. Variation in spawning site selection of the mummichog, Fundulus heteroclitus. Copeia 1984:522-525.
Able, K. W. and M. Castagna. 1975. Aspects of an undescribed reproductive behavior in Fundulus heteroclitus (Pisces: Cyprinodontidae) from Virginia. Ches. Sci. 16: 282-284.
Able, K.W., C.W. Talbot and J.K. Shisler. 1983. The spotfin killifish, Fundulus luciae, is common in New Jersey salt marshes. Bull. New Jersey Acad. Sci. 28:7-11.
Allen, D.M. and D.L. Barker. 1990. Interannual variations in larval fish recruitment to estuarine epibenthic habitats. Mar. Ecol. Prog. Ser. 63:113-125.
Allen, D.M., S.K. Service and M.V. Ogburn-Matthews. 1992. Factors influencing the collection of estuarine fishes. Trans. Am. Fish. Soc. 121:234-244.
Alon, N.C. and S. E. Stancyk. 1982. Variation in life-history patterns of the grass shrimp Palaemonetes pugio in two South Carolina estuarine systems. Mar. Biol. 68:265-276.
Archambault, J.A. and R.J. Feller. 1991. Diel variations in gut fullness of juvenile spot, Leiostomus xanthurus (Pisces). Estuaries 14:94-101.
Arnoldi, D.C., W.H.Herke and E.J. Clairain. 1974. Estimate of growth rate and length of stay in a marsh nursery of juvenile Atlantic croaker, Micropogon undulatus, "sandblasted" with fluorescent pigment. Gulf Caribb. Fish. Inst. Proc. 26:158-172.
Baker-Dittus, A.M. 1978. Foraging patterns of three sympatric killifish. Copeia 1978:383-389.
Baltz, D.M., C. Rakocinski and J.W. Fleeger. 1993. Microhabitat use by marsh- edge fishes in a Louisiana estuary. Env. Biol. Fish. 36:109-126.
Bass, R.J. and J.W. Avault. 1975. Food habits, length-weight relationships, condition factor and growth of juvenile red drum, Sciaenops ocellata, in Louisiana. Trans. Am. Fish. Soc. 1975:35-45.
Beckman, D.W. and J.M. Dean. 1984. The age and growth of young-of -the-year spot, Leiostomus xanthurus Lacapede, in South Carolina. Estuaries 7:487-496.
Bell, S.S. 1979. Short- and long-term variation in a high marsh meiofauna community. Est. Coast. Mar. Sci. 9:331-350.
Bell, S.S. 1982. On the population biology and meiofaunal characteristics of Manyunkia aesturina (Polychaeta: Sabellidae: Fabricinae) from a South Carolina salt marsh. Est. Coast. Shelf Sci. 14:215-221.
Bell, S.S. & B.C. Coull. 1978. Field evidence that shrimp predation regulates meiofauna. Oecologia 35:141-148.
Bell, S.S., M. C. Watzin, & B.C. Coull. 1978. Biogenic structure and its effect on the spatial heterogeneity of meiofauna in a salt marsh. J. Exp. Mar. Biol. Ecol. 35:99-107.
Boesch, D.F. and R.E. Turner. 1984. Dependence of fishery species on salt marshes: the role of food and refuge. Estuaries 7:460-468.
Boothby, R.N. and J.W. Avault. 1971. Food habits, Length-weight relationship, and condition factor of the red drum (Sciaenops ocellata) in Southeastern Louisiana. Trans. Am. Fish. Soc. 100:290-295.
Butner, A. and B.H. Brattstrom. 1960. Local movements in Menidia and Fundulus. Copeia 1960:139-141.
Byrne, D.M. 1978. Life history of the spotfin killifish, Fundulus luciae (Pisces: Cyprinodontidae), in Fox Creek Marsh, Virginia. Estuaries 1: 211-227.
Cadigan, K.M. and P.E. Fell. 1985. Reproduction, growth, and feeding habits of Menidia menidia (Atherinidae) in a tidal marsh-estuarine system in southern New England. Copeia 1985:21-26.
Cain, R. L. and J.M. Dean. 1976. Annual occurrence, abundance and diversity of fishes in a South Carolina intertidal creek. Mar. Biol. 36:369-379.
Chambers, R.M. and W.E. Odum. 1990. Porewater oxidation, dissolved phosphate and the iron curtain: iron-phosphorous relations in tidal freshwater marshes. Biogeochemistry 10:37-52.
Chick, J.H., F. Jordan, J.P. Smith, and C.C. McIvor. 1992. A comparsion of four enclosure traps and methods used to sample fishes in aquatic macrophytes. J. Freshw. Ecol. 7:353-361.
Coull, B.C. 1985. Long-term variability of estuarine meiobenthos: an 11 year study. Mar. Ecol. Prog. Ser. 24:205-218.
Coull, B.C. & R.J. Feller. 1988. Site-to-site variability in abundance of meiobenthic copepods along a tidal gradient over 24 hours. Hydrobiologia 167/168: 477-483.
Coull, B.C., S.S. Bell, A.M. Savory, & B.W. Dudley. Zonation of meiobenthic copepods in a southeastern United States salt marsh. Est. Coast. Shelf Sci. 9:181- 188.
Crabtree, R.E. and D.P. Middaugh. 1982. Oyster shell size and the selection of spawning sites by Chasmodes bosquianus, Hypleurochilus geminatus, Hypsoblennius ionthas (Pisces, Blenniidae) and Gobiosoma bosci (Pisces, Gobiidae) in two South Carolina estuaries.Estuaries 5:150-155.
Dahlberg, M.D. and E.P. Odum 1970. Annual cycles of species occurence, abundance, and diversity in Georgia estuarine fish populations. Am. Midl. Nat. 83:382-392.
Diaz, R.J., D.F. Boesch, J.L. Haver, C.A. Stone, and K. Munson. 1978. Part II: Aquatic biology - benthos. p. 18-54 in Habitat development field investigations, Windmill Point marsh development site, James River, Virginia. U.S. Army Waterways Exp. Stn. Tech. Rep. D-77-23.
Dieter, C.D., C.R. Berry and B. Kolterman. 1991. Fish enclosures for research in marshes. Wetlands 11:173-177.
DiMichele, L. and M.H. Taylor. 1980. The environmental control of hatching in Fundulus heteroclitus. J. Exp. Zool. 214:181-187.
Ellis, M.J. & B.C. Coull. 1989. Fish predation on meiobenthos: field experiments with juvenile spot Leiostomus xanthurus Lacepede. J. Exp. Mar. Biol. Ecol. 130:19-32.
Eskin, R.A. & B.C. Coull. 1987. Seasonal and three-year variability of meiobenthic nematode populations at two estuarine sites. Mar. Ecol. Prog. Ser. 41:295-303.
Everett, R.A. and G.M. Ruiz. 1993. Coarse woody debris as a refuge from predation in aquatic communities: an experimental test. Oecologia 93:475-486.
Feller, R.J., B.C. Coull & B.T. Hentschel. 1990. Meiobenthic copepods: tracers of where juvenile Leiostomus xanthurus (Pisces) feed? Can J. Fish Aquat. Sci. 47:1913-1919.
Fitz, H.C. and R.G. Weigert. 1991. Utilization of the intertidal zone of a salt marsh by the blue crab Callinectes sapidus: density, return, frequency, and feeding habits. Mar. Ecol. Prog. Ser. 76:249-260.
Fitzhugh, G.R. & J.W. Fleeger. 1985. Goby (Pisces: Gobiidae) interactions with meiofauna and small macrofauna. Bull. Mar. Sci. 36:436-444.
Fleeger, J.W. 1985. Meiofaunal densities and copepod species composition in a Louisiana, U.S.A., estuary. Trans. Am. Microsc. Soc. 104:321-332.
Fleeger, J.W., M.A. Palmer, & E.B. Moser. 1990. On the scale of aggregation of meiobenthic copepods on a tidal mudflat. Mar. Ecol. P.S.Z.N. I: 11:227-237.
Ford, T.E. and E.Mercer. 1986. Density, size distribution and home range of American eels, Anguilla rostrata, in a Massachusetts salt marsh. Env. Biol. Fishes 17:309-314.
Fritz, E.S., W.H. Meredith and V.A. Lotrich. 1975. Fall and Winter movements and activity level of the mummichog, Fundulus heteroclitus, in a tidal creek. Ches. Sci. 16:211-215.
Giles, J. H. and G. Zamora. 1973. Cover as a factor in habitat selection by juvenile brown (Penaeus aztecus) and white (P. setiferus) shrimp. Trans. Am. Fish. Soc. 102:144-145.
Gilmore, R. G., D.W. Cooke and C.J. Donohoe. 1983. A comparison of the fish populations and habitat in open and closed salt marsh impoundments in East- Central Florida. Northeast Gulf. Sci. 5:25-37.
Goulden, C.E., 1971. Environmental control of the abundance and distribution of the chydorid cladocera. Limnol. and Oceanog. 16: 320- 331.
Greeley, M.S. 1984. Spawning by Fundulus pulvereus and Adinia xenica (Cyprinodontidae) along the Alabama Gulf coast is associated with the semilunar tidal cycles. Copeia 1984:797-800.
Greeley, M.S. and R. MacGregor. 1983. Annual and semilunar reproductive cycles of the Gulf killifish, Fundulus grandis, on the Alabama Gulf coast. Copeia 1983:711-718.
Hackney, C.T. and A. de la Cruz. 1981. Some notes on the macrofauna of an oligohaline tidal creek in Mississippi. Bull. Mar. Sci. 31:658-661.
Harrington, R. W. 1959. Delayed hatching in stranded eggs of marsh killifish, Fundulus confluentus. Ecology 40:430-437
Harrington, R.W. and E.S. Harrington. 1960. Food of larval and young tarpon, Megalops atlantica. Copeia 1960:311-319.
Harrington, R.W. and E.S. Harrington. 1961. Food selection among fishes invading a high subtropical salt marsh: from onset of flooding through the progress of a mosquito brood. Ecology 42:646-666.
Harrington, R.W. and E.S. Harrington. 1982. Effects on fishes and their forage organisms of impounding a Florida salt marsh to prevent breeding by salt marsh mosquitoes. Bull. Mar.Sci. 32:523-531.
Harvey, J.W. and W.E.Odum. 1990. The influence of tidal marshes on upland groundwater discharge to estuaries. Biogeochemistry 10:217-236.
Hayden, B.P., R.D. Dueser, J.T. Callahan, and H.H. Shugart. 1991. Long-term research at the Virginia Coast Reserve. Bioscience 41:310-318.
Heard, R.W. 1975. Feeding habits of white catfish from a Georgia estuary. Florida Sci. 38:20-28.
Herke, W.H., P.M. Yakupzack and W.G. Perry. 1977. A trap for use on tidal weirs and streams. Northeast Gulf Sci. 1:34-38.
Herke, W.H., M.W. Wengert and M.E. LaGory. 1987. Abundance of young brown shrimp in natural and semi-impounded marsh nursery areas: relation to temperature and salinity. Northeast Gulf Sci. 9:9-28.
Hettler, W.F. 1989. Nekton use of regularly-flooded saltmarsh cordgrass habitat in North Carolina, USA. Mar. Ecol. Prog. Ser. 56:111-118.
Hodson, R.G., J.O. Hackman and C.R. Bennett. 1981. Food habits of young spots in nursery areas of the Cape Fear River Estuary, North Carolina. Trans. Am. Fish. Soc. 110:495-501.
Hoffman, J.A., J. Katz, & M. D. Bertness. 1984. Fiddler crab deposit-feeding and meiofaunal abundance in salt marsh habitats. J. Exp. Mar. Biol. Ecol. 82:161-174.
Jorgenson, S.C. 1969. A Georgia record for the Cyprinodontid fish, Fundulus luciae. Ches. Sci. 10:65.
Kilby, J.D. 1955. The fishes of two Gulf coastal marsh areas of Florida. Tulane Stud. Zool. 2:175-247.
Kjelson, M.A. and G.N. Johnson. 1973. Description and evaluation of a portable drop-net for sampling nekton populations. Proc. 27th Ann. Conf. Southeast. Assoc. Game Fish. Comm:653-652.
Kleypas, J. and J. M. Dean. 1983. Migration and feeding of the predatory fish, Bairdiella chrysura Lacepede, in an intertidal creek. J. Mar. Biol. Ecol. 72:199- 209.
Kneib, R.T. 1978. Habitat, diet, reproduction and growth of the spotfin killifish Fundulus luciae, from a North Carolina salt marsh. Copeia 1978: 164-168.
Kneib, R.T. 1981. Size-specific effects of density on the growth, fecundity and mortality of the fish Fundulus heteroclitus in an intertidal salt marsh. Mar. Ecol. Prog. Ser. 6:203-212.
Kneib, R.T. 1982. The effects of predation by wading birds (Ardeidae) and blue crabs (Callinectes sapidus) on the population size structure of the common mummichog, Fundulus heteroclitus. Est. Coast. Mar. Sci. 14:159-165.
Kneib, R.T. 1984. Patterns in the utilization of the intertidal salt marsh by larvae and juveniles of Fundulus heteroclitus (Linnaeus) and Fundulus luciae (Baird). J. Exp. Mar. Biol. Ecol. 83:41-51.
Kneib, R.T. 1986a. The role of Fundulus heteroclitus in salt marsh trophic dynamics. Am. Zool. 26:259-269.
Kneib, R.T. 1986b. Size-specific patterns in the reproductive cycle of the killifish, Fundulus heteroclitus (Pisces: Fundulidae) from Sapelo Island, Georgia. Copeia 1986:342-351.
Kneib, R.T. 1987a. Predation risk and use of intertidal habitats by young fishes and shrimp. Ecology 68: 379-386.
Kneib, R.T. 1987b. Seasonal abundance, distribution and growth of postlarval and juvenile grass shrimp (Palaemonetes pugio) in a Georgia, USA, salt marsh. Marine Biology 96: 215-223.
Kneib, R.T. 1991. Flume weir for quantitative collection of nekton from vegetated intertidal habitats. Mar. Ecol. Prog. Ser. 75:29-38.
Kneib, R.T. 1993. Growth and mortality in successive cohorts of fish larvae within an estuarine nursey. Mar. Ecol. Prog. Ser. 94:115-127.
Kneib, R.T. and A.E. Stiven. 1978. Growth, reproduction and feeding of Fundulus heteroclitus (L.) on a North Carolina salt marsh. J. Exp. Mar. Biol. Ecol. 31:121- 140.
Kneib, R.T. and J.H. Parker. 1991. Gross conversion efficiencies of mummichog and spotfin killifish larvae from a Georgia salt marsh. Trans. Am. Fish. Soc. 120:903-809.
Kushlan, J.A. 1976. Environmental stability and fish community diversity. Ecology 57:821-825.
Kushlan, J.A. 1981. Sampling characteristics of enclosure fish traps. Trans. Am. Fish. Soc. 110:557-562.
Lee, D.S., C.R. Gilbert, C.H. Hocutt, R.E. Jenkins, D.E. McAllister, and J.R. Stauffer, Jr. 1980. Atlas of North American Freshwater Fishes. North Carolina State Museum of Natural History, 867 pp.
Lorio, W.J. and H.E. Schafer. 1965. A food habit study of the spotted seatrout, Cynoscion nebulosus, in the Biloxi Marsh area, Louisiana. Proc. 19th Ann. Conf. S.E. Game and Fish. Comm:289-295.
Lotrich, V.A. 1975. Summer home range and movements of Fundulus heteroclitus (Pisces: Cyprinodontidae) in a tidal creek. Ecology 56:191-198.
McIvor, C.C. and W.E. Odum. 1986. The flume net: a quantitative method for sampling fishes and macrocrustaceans on tidal marsh surfaces. Estuaries 9: 219-224.
McIvor, C.C. and W.E. Odum. 1988. Food, predation risk, and microhabitat selection in a marsh fish assemblage. Ecology 69:1341-1351.
Mense, D.J. and E.L. Wenner. 1989. Distribution and abundance of early life history stages of the blue crab, Callinectes sapidus, in tidal marsh creeks near Charleston, South Carolina. Estuaries 12:157-168.
Meredith, W.H. and V.A. Lotrich. 1979. Production dynamics of a tidal creek population of Fundulus heteroclitus (Linnaeus). Est. Coast. Mar. Sci. 8:99-118.
Minello. T.J. and R.J. Zimmerman. 1983. Fish predation on juvenile brown shrimp, Penaeus aztecus Ives: the effect of simulated Spartina structure on predation rates. J. Exp. Mar. Biol. Ecol.72:211-231.
Minello, T.J. and R.J. Zimmerman. 1985. Differential selection for vegetative structure between juvenile brown shrimp (Penaeus aztecus) and white shrimp (P. setiferus), and implications in predator-prey relationships. Est. Coast. Shelf. Sci. 20:707-716.
Minello, T.J. and R.J. Zimmerman. 1992. Utilization of natural and transplanted Texas salt marshes by fish and decapod crustaceans. Mar. Ecol. Prog. Ser. 90:273-285
Minello, T.J., R.J. Zimmerman and E.X. Martinez. 1989. Mortality of young brown shrimp Penaeus aztecus in estuarine nurseries. Trans. Am. Fish. Soc. 118:693- 708.
Munson, K.E. 1980. A study of the distribution and habitat preference of meiobenthos of a freshwater tidal marsh on the James River, Virginia. M.S. Thesis, George Washington University, Washington, D.C.
Neill, C. and R.E. Turner. 1987. Comparison of fish communities in open and plugged backfilled canals in Louisiana coastal marshes. N. Am. J. Fish. Manage. 7:57-62.
Nelson, A.L. & B.C. Coull. 1989. Selection of meiobenthic prey by juvenile spot (Pisces): an experimental study. Mar. Ecol. Prog. Ser. 53:51-57.
NOAA. 1991. Climatological Data - Virginia, July 1991. Vol. 101. No. 7.
Norcross, D.L. and D. Hata. 1990. Seasonal composition of finfish in waters behind the Virginia barrier islands. Va. J. Sci.: 441-461.
Odum, W.E., T.J. Smith III, J.K. Hoover, & C.C. McIvor, 1984. The ecology of tidal freshwater marshes of the United States east coast: a community profile. U.S. Fish Wildl. Serv. FWS/OBS-83/17. 177p.
Orth, R.J. and J. van Montfrans. 1987. Utilization of a seagrass meadow and tidal marsh creek by blue crabs Callinectes sapidus. I. Seasonal and annual variations in abundance with emphasis on post-settlement juveniles. Mar. Ecol. Prog. Ser. 41:283-294.
Osenga, G.A. & B.C. Coull. 1983. Spartina alterniflora Loisel root structure and meiofaunal abundance. J. Exp. Mar. Biol. Ecol. 67:221-224.
Palmer, M.A. 1980. Variation in life-history patterns between intertidal and subtidal populations of the meiobenthic copepod Microarthridion littorale. Mar. Biol. 60:159-165.
Palmer, M.A. & R.R. Brandt. 1981. Tidal variation in sediment densities of marine benthic copepods. Mar. Ecol. Prog. Ser. 4:207-212.
Peckarsky, B.L., P.R. Fraissinet, M.A. Penton, & D.J. Conklin, Jr. 1990. Freshwater Macroinvertebrates of Northeastern North America. Cornell University Press, Ithaca, New York, 442 pp.
Pennak, R.W., 1989. Freshwater Invertebrates of the United States, 2nd Ed., John Wiley and Sons, Inc., New York, 628 pp.
Peterson, M.S. and S.T. Ross. 1991. Dynamics of littoral fishes and decapods along a coastal river-estuarine gradient. Est. Coast Shelf. Sci. 33:467-483.
Phillips, F.E. & J. W. Fleeger. 1985. Meiofauna meso-scale variability in two estuarine habitats. Est. Coast. Shelf. Sci. 21:745-756.
Rader, D.N. 1984. Salt-marsh benthic invertebrates: small-scle patterns of distribution and abundance. Estuaries 7:413-420.
Rakocinski, C. F., D.M. Baltz and J.W. Fleeger. 1992. Correspondence between environmental gradients and the community structure of marsh-edge fishes in a Louisiana estuary. Mar. Ecol. Prog. Ser. 80:135-148.
Richards, C.E. and R.L. Bailey. 1967. Occurence of Fundulus luciae, spotfin killifish, on the seaside of Virginia's Eastern Shore. Ches. Sci. 8: 204 - 205.
Richards, C.E. and M. Castagna. 1970. Marine fishes of Virginia's Eastern Shore (inlet and marsh, seaside waters). Ches. Sci. 11: 235-248.
Rickards, W.L. 1968. Ecology and growth of juvenile tarpon, Megalops atlanticus, in a Georgia salt marsh. Bull. Mar. Sci 18:220-239.
Rogers, B.D. 1985. A small push-otter trawl for use in shallow marshes. N.Am.J. Fish. Manage.5:411-415.
Rogers, B.D., W.H. Herke and E.E. Knudsen. 1992. Effects of three different water-control structures on the movements and standing stocks of coastal fishes and macrocrustaceans. Wetlands 12:106-120.
Rogers, B.D., R.F. Shaw, W.H. Herke and R.H. Blanchet. 1993. Recruitment of postlarval and juvenile brown shrimp (Penaeus aztecus Ives) from offshore to estuarine waters of the northwestern Gulf of Mexico. Est Coast. Shelf Sci. 36:377-394.
Rogers, S.G., T.E. Targett and S.B. Van Sant. 1984. Fish-nursery use in Georgia salt-marsh estuaries: the influence of Springtime freshwater conditions. Trans. Am. Fish. Soc. 113:595-606.
Rountree, R.A. and K.W. Able. 1992a. Fauna of polyhaline subtidal marsh creeks in southern New Jersey: composition, abundance and biomass. Estuaries 15:171- 185.
Rountree, R.A. and K.W. Able. 1992b. Foraging habits, growth, and temporal patterns of salt-marsh creek habitat use by young-of-year summer flounder in New Jersey. Trans. Am. Fish. Soc. 121:765-776.
Rountree, R.A. and K.W. Able. 1993. Diel variation in decapod crustacean and fish assemblages in New Jersey polyhaline marsh creeks. Est. Coast. Shelf Sci. 37:181-201.
Rozas, L.P. 1992. Comparison of nekton habitats associated with pipeline canals and natural channels in Louisiana salt marshes. Wetlands 12:136-146.
Rozas, L.P. 1993. Bottomless lift net for quantitatively sampling nekton on intertidal marshes. Mar. Ecol. Prog. Ser. 89:287-292.
Rozas, L.P. and C.T. Hackney. 1983. The importance of oligohaline estuarine wetland habitats to fisheries resources. Wetlands 3:77-89.
Rozas, L.P. and C.T. Hackney. 1984. Use of oligohaline marshes by fishes and macrofaunal crustaceans in North Carolina. Estuaries 7:213-224.
Rozas, L.P. and W.E. Odum. 1987a. Fish and macrocrustacean use of submerged plant beds in tidal freshwater marsh creeks. Mar. Ecol. Prog. Ser. 38: 101-108.
Rozas, L.P. and W.E. Odum. 1987b. The role of submerged aquatic vegetation in influencing the abundance of nekton on contiguous tidal freshwater marshes. J. Exp. Mar. Biol. Ecol. 114:289-300.
Rozas, L.P. and W.E. Odum. 1987c. Use of tidal freshwater marshes by fishes and macrofaunal crustaceans along a marsh stream-order gradient. Estuaries 10:36-43.
Rozas, L.P. and W.E. Odum. 1988. Occupation of submerged aquatic vegetation by fishes: testing the roles of food and refuge. Oecologia 77:101-106.
Rozas, L.P., C.C. McIvor and W.E. Odum. 1988. Intertidal rivulets and creekbanks: corridors between tidal creeks and marshes. Mar. Ecol. Prog. Ser. 47:303- 307.
Rozas, L. P. and M.W. LaSalle. 1990. A comparison of the diets of Gulf killifish, Fundulus grandis Baird and Girard, entering and leaving a Mississippi brackish marsh. Estuaries 13:332-336.
Rozas, L.P. and D.J. Reed. 1993. Nekton use of marsh-surface habitats in Louisiana (USA) deltaic salt marshes undergoing submergence. Mar. Ecol. Prog. Ser. 96:147-157.
Ryer, C.H. 1987. Temporal patterns of feeding by blue crabs (Callinectes sapidus) in a tidal-marsh creek and adjacent seagrass meadow in the lower Chesapeake Bay. Estuaries 10:136-140.
Serafy, J.E., R.M. Harrell, & J.C. Stevenson. 1988. Quantitative sampling of small fishes in dense vegetation: Design and field testing of portable "pop-nets". J. Appl. Ichthyol. 4: 149-157.
Shenker, J.M. and J.M. Dean. 1979. The utilization of an intertidal salt marsh creek by larval and juvenile fishes: abundance diversity and temporal variation. Estuaries 2:154-163.
Shields, M.A. and C.H. Hayes. 1983. Occurence and habitat preference of Fundulus luciae (Baird) (Pisces: Cyprinodontidae) on a Southeastern North Carolina salt marsh. Brimleyana 9: 141-144.
Smith, L.D. & B.C. Coull. 1987. Juvenile spot (Pisces) and grass shrimp predation on meiobenthos in muddy and sandy substrata. J. Exp. Mar. Biol. Ecol. 105:123- 136.
Sogard, S.M. and K.W. Able. 1991. A comparison of eelgrass, sea lettuce macroalgae, and marsh creeks as habitats for epibenthic fishes and decapods. Est. Coast. and Shelf Sci. 33:501-519.
Sokal, R.R. & J. Rohlf. 1981. Biometry. 2nd. Ed. W.H. Freeman and Co. New York, 859 pp.
Subrahmanyam, C.B. and S.H. Drake. 1975. Studies on the animal communities in two north Florida salt marshes Part I. fish communities. Bull. Mar. Sci. 25:445- 465.
Subrahmanyam, C.B. and C.L. Coultas. 1980. Studies on the animal communities in two north Florida salt marshes Part III. seasonal fluctuations of fish and macroinvertebrates. Bull. Mar. Sci. 30:790-818.
Talbot, C.W. and K.W. Able. 1984. Composition and distribution of larval fishes in New Jersey high marshes. Estuaries 7:434-443.
Talbot, C.W., K.W. Able and J.K. Shisler. 1986. Fish species composition in New Jersey salt marshes: effects of marsh alterations for mosquito control. Trans. Am. Fish. Soc. 115:269-278.
Taylor, M.H. 1986. Environmental and endrocine influences on reproduction of Fundulus heteroclitus. Amer. Zool. 26:159-171.
Taylor, M.H. and L. DiMichele. 1980. Ovarian changes during the lunar spawning cycle of Fundulus heteroclitus. Copeia 1980:118-125.
Taylor, M.H. and L. DiMichele. 1983. Spawning site utilization in a Delaware population of Fundulus heteroclitus (Pisces: Cyprinodontidae). Copeia 1983:719-725.
Taylor, M.H., L. DiMichele and G.J. Leach. 1977. Egg stranding in the life cycle of the mummichog, Fundulus heteroclitus. Copeia 1977:397-399.
Taylor, M.H., G.J. Leach, L. DiMichele, W.M. Levitan and W.F. Jacob. 1979. Lunar spawning cycle in the mummichog, Fundulus heteroclitus (Pisces: Cyprinodontidae). Copeia 1979:291-297.
Thayer, G.W., H.H. Stuart, W.J. Kenworthy, J.F. Ustach and A. B. Hall. 1979. Habitat values of salt marshes, mangroves, and seagrasses for aquatic organisms. pp. 235-247 in P.E. Greeson, J.R. Clark and J.E. Clark, (Eds.) Wetland Functions and Values: The State of our Understanding. AWRA Tech. Publ. TPS79-2.
Thomas, J.L., R.J. Zimmerman and T.J. Minello. 1990. Abundance patterns of juvenile blue crabs (Callinectes sapidus) in nursery habitats of two Texas bays. Bull. Mar. Sci. 46:115-125.
Valiela, I., J.E. Wright, J.M. Teal and S. B. Volkmann. 1977. Growth, production and energy transformations in the salt-marsh killifish Fundulus heteroclitus. Mar. Biol. 40:135-144.
Vince, S., I. Valiela, N. Backus and J.M. Teal. 1976. Predation by the salt marsh killifish Fundulus heteroclitus (L.) in relation to prey size and habitat structure: consequences for prey distribution and abundance. J. Exp. Mar. Biol. Ecol. 23:255-266.
Weinstein, M.P. 1979. Shallow marsh habitats as primary nurseries for fishes and shellfish, Cape Fear River, North Carolina. Fish. Bull. 77:339-357.
Weinstein, M.P. 1983. Population dynamics of an estuarine-dependent fish, the spot (Leiostomus xanthurus) along a tidal creek-seagrass meadow coenocline. Can J. Fish Aquat. Sci. 40:1633-1638.
Weinstein, M.P. and H.A. Brooks. 1983. Comparative ecology of nekton residing in a tidal creek and adjacent seagrass meadow: community composition and structure. Mar. Ecol. Prog. Ser. 12:15-27.
Weinstein, M.P and R.W. Davis. 1980. Collection efficiency of seine and rotenone samples from tidal creeks, Cape Fear River, North Carolina. Estuaries 3:98-105.
Weinstein, M.P. and M. P. Walters. 1981. Growth, survival and production in young-of-year populations of Leiostomus xanthurus Lacepede residing in tidal creeks. Estuaries 4:185-197.
Weinstein, M.P., L. Scott, S. P. O'Neil, R.C. Siegfried and S.T. Szedlmayer. 1984. Population dynamics of spot, Leiostomus xanthurus, in polyhaline tidal creeks of the York River Estuary, Virginia. Estuaries 7:444-450.
Weinstein, M.P., S.L. Weiss and M.F. Walters. 1980. Multiple determinants of community structure in shallow marsh habitats, Cape Fear River Estuary, North Carolina, USA. Mar. Biol. 58:227-243.
Weisberg, S.B. 1986. Competition and coexistence among four estuarine species of Fundulus. Amer. Zool. 26:249-257.
Weisberg, S.B. and V.A. Lotrich. 1986. Food limitation of a Delaware salt marsh population of the mummichog, Fundulus heteroclitus (L.). Oecologia 68:163-173.
Weisberg, S.B., R.Whalen and V.A. Lotrich. 1981. Tidal and diurnal influence on food consumption of a salt marsh killifish Fundulus heteroclitus. Mar. Biol. 61:243-246.
Welsh, B.L. 1975. The role of grass shrimp, Palaemonetes pugio, in a tidal marsh ecosystem. Ecology 56: 513 - 530.
West, D.L. and A.H. Williams. 1986. Predation by Callinectes sapidus (Rathbun) within Spartina alterniflora (Loisel) marshes. J. Exp. Mar. Biol. Ecol. 100:75-95.
Wilson, K.A., K.W. Able and K.L. Heck. 1990a. Habitat use by juvenile blue crabs: a comparison among habitats in southern New Jersey. Bull. Mar. Sci. 46:105-114.
Wilson, K.A., K.W. Able and K.L. Heck. 1990b. Predation rates on juvenile blue crabs in estuarine nursery habitats: evidence for the importance of macroalgae (Ulva lactuca). Mar. Ecol. Prog. Ser. 58:243-251.
Wirjoatmodjo, S. and T.J. Pitcher. 1984. Flounders follow the tides to feed: evidence from ultrasonic tracking in an estuary. Est. Coast. Shelf Sci. 19:231-241.
Worgan, J.P. and G.J. Fitzgerald. 1981. Diel activity and diet of three sympatric sticklebacks in tidal salt marsh pools. Can J. Zool. 59:2375-2379.
Yozzo, D.J. & W.E. Odum. 1993. Fish predation on epiphytic microcrustacea in Tivoli South Bay, a Hudson River tidal freshwater wetland. Hydrobiologia 57:37-46.
Zimmerman, R.J. and T.J. Minello. (1984). Densities of Penaeus aztecus, Penaeus setiferus and other natant macrofauna in a Texas salt marsh. Estuaries 10: 36-43.
Estuarine-dependent nekton were sampled from three subtidal stations in Phillips Creek (see map in Chapter III) during summer 1993. Project objectives were 1) to determine species composition, abundance and distribution of nekton within Phillips Creek. 2) to document food habits of selected nekton species, and 3) to determine the feasibility of various gear types with regard to a proposed nekton monitoring program at the VCR-LTER.
SAMPLING GEAR
PHYSICO-CHEMICAL PARAMETERS
A total of 23 species were present in seine collections; 11 species were caught in gill-nets. Seine collections were dominated by Sciaenids (spot and silver perch) and blue crabs. Demersal species were well-represented in seine collections. Spot were abundant in seine collections from June through mid-July; silver perch dominated catches from mid-July through mid-August. Sciaenids were most abundant at the lower and middle creek stations, while white mullet and Atlantic menhaden were abundant at the upper creek station; Gill-nets were effective in sampling schooling species such as Atlantic menhaden and mullet. Blue crabs were numerically dominant in gill-nets, but may have been attracted to the carcasses of trapped fishes. Juvenile sandbar sharks were effectively collected with larger (7.63 cm ) mesh gill-nets.
Small crustraceans (copepods, larval and juvenile decapods, and juvenile daggerblade grass shrimp) constituted a significant percentage of food items for juvenile spot, silver perch, and horse-eye jacks . Summer flounder preyed heavily upon grass shrimp. Juvenile sandbar sharks preyed upon blue crabs, various fishes and mantis shrimp. Overall (exclusive of sharks) 32% of total fishes collected during incoming tides had empty guts, while less than 15% of fishes collected on outgoing tides went hungry.
Seine collections were effective at collecting a wide variety and size range of species, mostly with demersal habitat preferences. Seining was limited to low tide or shallow water conditions. Gill nets were effective at collecting schooling mid-water species and could be deployed in deeper water (ie. creek mouths) Short term net sets (1.5 - 4 hr) were not productive. Longer sets (18 - 24 hr) yielded many more specimens but obscured information on time and tide stage of collection. Crab damage to nets and specimens was significant during long sets.
common biomass TL range Species name n (g) (mm) Callinectes sapidus blue crab 183 9126.24 34 - 145* Mugil cerema white mullet 172 2369.80 100 - 200 Brevoortia tyrannus Atlantic menhaden 123 964.99 67 - 124 Leiostomus xanthurus spot 58 389.46 75 - 98 Carcharhinus plumbeus sandbar shark 23 20535.00 525 - 680 Caranx latus horse-eye jack 21 121.00 N/A Bairdiella chrysoura silver perch 17 415.38 81 - 220 Fundulus majalis striped killifish 5 51.94 93 - 98 Malaclemys t. terrapin diamondback terrapin 4 1265.00 128 - 145** Paralichthys dentatus summer flounder 4 336.87 34 - 172 Synodus foetens inshore lizardfish 1 20.25 156 ___ ________ ______ TOTALS: 611 35595.93 34 - 680 * Carapace width (mm) ** Carapace length (mm)
common biomass TL range Species name n (g) (mm) Leiostomus xanthurus spot 794 2093.98 18 - 108 Callinectes sapidus blue crab 376 17326.98 18 - 165* Bairdiella chrysoura silver perch 206 510.75 28 - 89 Brevoortia tyrannus Atlantic menhaden 55 504.63 33 - 123 Paralichthys dentatus summer flounder 54 350.20 42 - 147 Caranx latus horse-eye jack 44 182.73 32 - 112 Mugil cephalus striped mullet 31 52.75 37 - 65 Mugil cerema white mullet 20 180.87 47 - 178 Symphurus plagiusa blackcheek tonguefish 17 110.21 61 - 130 Synodens foetens inshore lizardfish 11 47.80 66 - 130 Opsanus tau oyster toadfish 10 299.96 40 - 80 Trinectes maculatus hogchoker 4 49.60 92 -123 Pomatomus saltatrix bluefish 4 23.53 71 - 96 Anguilla rostrata American eel 2 173.88 333 - 480 Fundulus majalis striped killifish 2 8.60 44 - 93 Lolliguncula brevis brief squid 2 8.60 45 - 85 Carcharhinus plumbeus sandbar shark 1 1750.00 712 Malaclemys t. terrapin diamondback terrapin 1 400 148** Alpheus heterochaelis snapping shrimp 1 1.50 62 Strongylura marina Atlantic needlefish 1 1.20 116 Cyprinodon variegatus sheepshead minnow 1 0.80 37 Squilla empusa mantis shrimp 1 0.66 42 Penaeus spp. shrimp 1 n/a 135 ____ _______ ______ TOTALS: 1640 24079.62 18 - 712 * Carapace width (mm) ** Carapace length (mm)Note: Mummichogs Fundulus heteroclitus, Atlantic silversides Menidia menidia and dagger-blade grass shrimp Palaemonetes pugio were abundant in most seine hauls; however, many individuals were not of sufficient size to be retained by the 9.5 mm mesh and are not included in this dataset.
 Total nekton abundance, upper, middle, and lower Phillips Creek
stations, summer 1993.
Total nekton abundance, upper, middle, and lower Phillips Creek
stations, summer 1993.
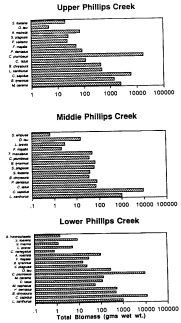 Total nekton biomass (gms wet wt.), upper, middle, and lower Phillips Creek
stations, summer 1993.
Total nekton biomass (gms wet wt.), upper, middle, and lower Phillips Creek
stations, summer 1993.
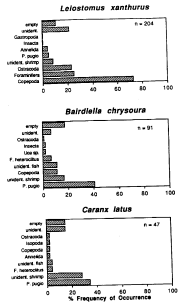 Gut contents of spot Leiostomus xanthurus, silver perch Bairdeilla
chrysoura, and horse-eye jack Caranx latus collected from Phillips
Creek, summer 1993.
Gut contents of spot Leiostomus xanthurus, silver perch Bairdeilla
chrysoura, and horse-eye jack Caranx latus collected from Phillips
Creek, summer 1993.
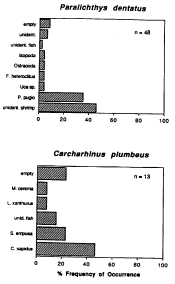 Gut contents of summer flounder Paralichthys dentatus and juvenile
sandbar sharks Carcharinus plumbeus collected from Phillips Creek,
summer 1993.
Gut contents of summer flounder Paralichthys dentatus and juvenile
sandbar sharks Carcharinus plumbeus collected from Phillips Creek,
summer 1993.
Nekton were collected monthly from a large permanent salt pond located in East Brownsville (see map in Chapter III), VCR-LTER from June - November 1991 in order to determine species composition and abundance of resident nekton . Pond dimensions were 92 x 52 m at its longest and widest sections. The pond was apparently flooded by seawater from adjacent Castle Ridge Creek only during extreme lunar or wind-driven tides.
SAMPLING GEAR
PHYSICO-CHEMICAL PARAMETERS
Six fish and up to three decapod species comprised the nekton community of the Brownsville salt pond. In general, nekton were abundant throughout the summer; few individuals were collected in November. Cyprinodonts dominated collections and were represented by mummichogs, sheepshead minnows, spotfin killifish, and striped killifish. Although mummichogs are abundant in nearly all salt marsh sub-environments, sheepshead minnows are apparently restricted to relatively large permanent to semi-permanent high marsh ponds. Sheepshead minnows were never taken from intertidal marsh locations during 1991 - 1993 ( Chapters 2 and 4). One individual was collected from Phillips Creek in Summer 1993 (Appendix I). Spotfin killifish were rare in pond collections, but may be locally abundant in upper intertidal marsh habitats (Chapters 2 and 4). Striped killifish may be abundant in shallow sub-tidal habitats, but are generally absent from the intertidal (Chapter 2 and 4).
Presence of striped killifish, Atlantic silversides, and striped mullet suggest that the pond is periodically, although infrequently, inundated by floodwaters, at which time exchange of marsh-dependent nekton may occur. Salt ponds may function as a nursery habitat for decapod crustaceans in addition to finfish, as evidenced by the presence of sub-adult and adult blue crabs, penaid shrimp and daggerblade grass shrimp.
JUNE - NOVEMBER 1991
common TL range Species name n (mm) Fundulus heteroclitus mummichog 2262 17 - 85 Cyprinodon variegatus sheepshead minnow 435 16 - 53 Fundulus majalis striped killifish 96 22 - 56 Callinectes sapidus blue crab 51 9 - 222* Menidia menidia Atlantic silverside 21 20 - 74 Fundulus luciae spotfin killifish 7 25 - 34 Mugil cephalus striped mullet 1 100 ___ _______ TOTALS : 2873 9 - 222 * Carapace width (mm)Note: Daggerblade grass shrimp Palaemonetes pugio were abundant in seine hauls from August - November, however, most were not of sufficient size to be retained by the 9.5 mm mesh and are not included in this dataset. In addition, two juvenile penaid shrimp were collected in August.
Speceis June July August September October November Fundulus heteroclitus 186.7 +88.9 217.3 +167.4 188.3 +47.5 128.3 +30.0 29.0 +4.2 1.0 +0.6 Cyprinodon variegatus 96.3 +29.8 113.3 +83.6 62.3 +3.7 41.7 +8.4 101.3 +65.9 2.0 +1.2 Fundulus majalis 23.3 +5.0 10.3 +6.0 42.7 +18.5 20.7 +7.8 6.0 +2.3 0 Callinectes sapidus 1.3 +0.8 0.3 +0.3 10.0 +4.6 4.3 +2.6 3.3 +1.7 0 Menidia menidia 3.7 +1.5 0 0 0 2.0 +0.6 0.3 +0.3 Fundulus luciae 1.7 +1.2 0 0.7 +0.7 0 0 0 Mugil cephalus 0.3 +0.3 0 0 0 0 0
Marsh - dependent nekton were collected from the mouth of Beaver Dam Creek on nine monthly sampling trips from July - October 1992 and May - September 1993. Project objectives were 1) to determine species composition and abundance of fishes within Beaver Dam Creek and 2) to document feeding habits of selected fish species.
SAMPLING GEAR
PHYSICO-CHEMICAL PARAMETERS (1993 only)
Eleven fish species were collected in gill-nets. Samples were dominated by white perch, largemouth bass, bluegill sunfish, and channel catfish.
Largemouth bass fed primarily upon small marsh-dependent nekton, including daggerblade grass shrimp and Fundulus spp. Many fish remains present in bass stomachs were digested beyond identification. Thirty-nine percent (39%) of bass examined preyed upon fish; of these, 95% of the total wet mass present in guts was comprised of fish remains. White perch primarily consumed amphipods (Gammarus fasciatus) and other small crustaceans and insects. Channel catfish consumed amphipods, megalopterans, and much vascular plant material, including the seeds of the emergent macrophyte Peltandra virginica.
Crab damage to nets and specimens was occasionally a problem. In addition, the nets tended to collect large amounts of macrophyte-derived seston, particularly in September and October when submersed vegetation beds were rapidly decomposing. This may have induced a gear avoidance response in some species.
common TL range Species name n (mm) Morone americana white perch 43 136 - 196 Micropterus salmoides largemouth bass 33 96 - 279 Ictalurus punctatus channel catfish 20 200 - 412 Lepomis macrochirus bluegill sunfish 19 66 - 186 Notemigonus crysoleucas golden shiner 16 104 - 226 Perca flavescens yellow perch 4 180 - 300 Dorosoma cepedianum gizzard shad 4 220 - 300 Ameiurus catus white catfish 3 235 - 238 Lepisosteus osseus longnose gar 1 722 Ameiurus nebulosus brown bullhead 1 250 Trinectes maculatus hogchoker 1 98 ___ _______ TOTALS: 145 66 - 722Note: Blue crabs Callinectes sapidus were frequently collected in gill nets but were not included in the dataset. Crabs may have been attracted to carcasses of trapped fishes.
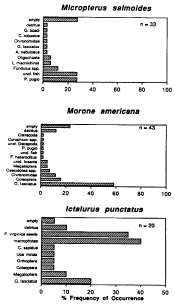 Food habits of largemouth bass Micropterus salmoides, white perch
Morone americana, and channel catfish Ictalurus punctatus
collected from Beaver Dam Creek, 1992 - 1993.
Food habits of largemouth bass Micropterus salmoides, white perch
Morone americana, and channel catfish Ictalurus punctatus
collected from Beaver Dam Creek, 1992 - 1993.
Benthic macroinvertebrates were sampled quarterly during 1993 in order to document abundance, distribution, and species composition of the intertidal macrofaunal community. Ceramic plates (17.78 cm diam., 5 cm depth) were placed upon the marsh surface at upper and lower intertidal locations. Sediment accumulated rapidly within collection plates which were readily colonized by marsh macrofauna. This method was developed after initial attempts at coring the soft, highly organic marsh substrate proved unreliable.
There was no significant difference in total abundance or biomass (gms. AFDW) between high and low marsh samples (ANOVA, p > 0.05). Total biomass and abundance of the four most abundant taxa (Caecidotea spp., G. fasciatus, tubificids and chironomids) did not differ significantly between high and low marsh locations, but did differ significantly among sampling dates (p < 0.05).
Total high marsh Total low marsh Platyhelminthes Turbellaria 2 1 Nematomorpha 3 6 Annelida Tubificidae 420 244 Mooreobdella spp. 5 0 Placobdella ornata 27 10 Arthropoda Chironomidae 59 112 Hemiptera 1 2 Enellagma spp. 0 1 Somatochlora spp. 4 2 Lepidoptera 2 0 Sialis spp. 49 0 Gammarus fasciatus 542 272 Corophium spp. 21 1 Caecidotea spp. 166 116 Sphaereum quadridentatus 16 1 Palaemonetes pugio 0 72 Rhithropanopeus harrisi 1 0 Callinectes sapidus 0 1 Gastropoda Gyraulus parvus 3 4 Physa integra 11 9 Pisidium spp. 156 3 ____ ___ TOTAL 1488 846
A survey of the macro-epifaunal community of water nymph (Najas minor) was conducted on three dates in 1993 (July 24, August 12, August 23) in a water nymph bed located at the confluence of Beaver Dam Creek and the Chickahominy River. Collections were obtained during estimated peak biomass of submersed vegetation with a 18.8 L Nalgene clear plastic cylinder which was rapidly pushed down into the plant bed, enclosing submersed vegetation and associated epiphytic animals. While resting just above the substrate, the cylinder was capped with a plastic lid and rapidly inverted. The entire sample (water and associated macrophytes and organisms) were washed thru a 1 mm mesh net in the field. All samples were collected at low tide; water depth within the bed was approximately 1 meter. Five replicate samples were taken on each of the three collection dates.
In the laboratory, macrophyte samples were washed to remove all epiphytic macrofauna. All material removed from plant stems and leaves was preserved in 10% neutral formalin with Rose Bengal. Washed plant material was weighed wet and oven dried at constant temperature (60 deg. C) for 48 hrs. Epiphytic organisms were counted, and identified to major taxon (fishes, crustaceans, and gastropods were identified to species if possible).
Analysis of the 15 samples yielded 788 invertebrates and 20 vertebrates (including 2 fish eggs). Six invertebrate taxa representing three major groups (Insecta, Crustacea, Gastropoda) comprised 94% of all organisms collected. Aquatic insect larvae comprised a significant portion of the epifauna. Chironomids and mayflies (Ephemeroptera) represented 13% of total organisms collected. Other insect taxa present in beds include the Zygopteran Enellagma and Trichopterans. Crustaceans were represented by the daggerblade grass shrimp Palaemonetes pugio and the amphipod Gammarus fasciatus. These two species are free-swimming and are widely distributed in tidal freshwater wetland sub-environments. Gastropods were represented in the epifauna by 5 species, two of which (Fossaria spp. and Gyraulus parvus) comprised 26% of the total organisms collected.
Several fish species were collected, despite the obvious deficiencies of the sampling technique for collecting rapidly swimming organisms. The structural complexity of the dense beds may have inhibited escape by small fishes and other free-swimming nekton (ie. P. pugio). All fishes captured were juveniles or young adults. Four unidentified fish larvae and two unidentified fish eggs were collected.
n INVERTEBRATES Tubificidae 5 unident. Dipteran 1 Chironomidae 76 Enellagma spp. 9 Trichoptera 7 Ephemeroptera 28 Hemiptera 2 Palaemonetes pugio 381 Rhithropanopeus harrisi 1 Callinectes sapidus 1 Gammarus fasciatus 51 Hydracarinae 2 Gyraulus parvus 58 Physa integra 10 Goniobasis spp. 1 Fossaria spp. 151 Pisidium spp. 5 ___ TOTAL 789 VERTEBRATES Anguilla rostrata 1 Fundulus diaphanus 7 Fundulus heteroclitus 1 Gambusia affinis holbrooki 1 Enneacanthus gloriosus 1 Lepomis macrochirus 1 Morone spp. 1 fish egg 2 unident. fish larvae 4 __ TOTAL 20